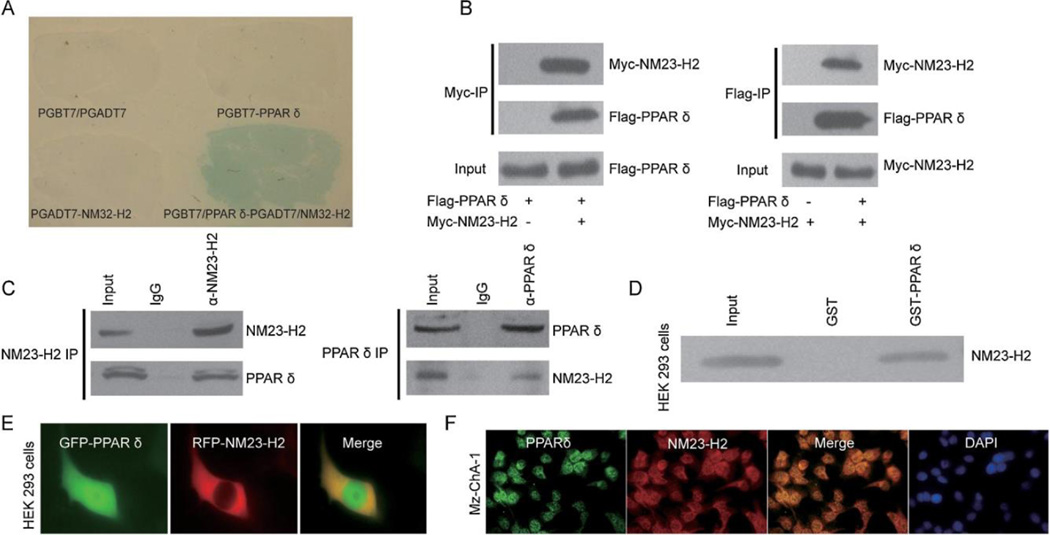
Fig. 2.
PPAR interacts with NM23-H2. (A) Yeast two-hybrid screening was performed using PPAR and a mouse kidney cDNA library. NM23-H2 was identified as a novel molecular partner that interacted with PPAR. (B) Co-immunoprecipitation experiments were performed in HEK293 cells transfected with pcDNA3-Myc-NM23-H2 and pcDNA3-Flag-PPAR. (C) Co-immunoprecipitation experiments were performed in normal rat cholangiocytes (NRC) to detect endogenous PPAR and NM23-H2 interaction. (D) A binding assay was performed using purified recombinant GST-PPAR protein and Myc-NM23-H2-expressing HEK293 cell extracts. (E) Co-localization of GFP-tagged PPAR and RFP-tagged NM23-H2 were tested in HEK293 cells (40×) transfected with plasmids encoding PPAR or NM23-H2. (F) Mz-ChA-1 cells were immunostained with PPAR and NM23-H2 antibodies. Overlap of PPAR (green) and NM23-H2 (red) staining was shown and nuclei were labelled with DAPI (blue). Images obtained with confocal microscopy (40×). PPAR, peroxisome proliferator-activated receptor; NM23-H2, CCA nucleoside diphosphate kinase alpha; NRC, cholangiocarcinoma. normal rat cholangiocytes.