Abstract
Osteosarcoma, the most common type of primary bone cancer, is the second highest cause of cancer-related death in pediatric patients. To understand the mechanisms behind osteosarcoma progression and to discover novel therapeutic strategies for this disease, a reliable and appropriate mouse model is essential. For this purpose, osteosarcoma cells need to be injected into the bone marrow. Previously, the intratibial and intrafemoral injection methods were reported; however, the major drawback of these methods is the potential leakage of tumor cells from the injection site during or after these procedures. To overcome this, we have established an improved method to minimize leakage in an orthotopic mouse model of osteosarcoma. By taking advantage of the anatomical benefits of the femur with less bowing and larger medullary cavity than those of the tibia, osteosarcoma cells are injected directly into the femoral cavity following reaming of its intramedullary space. To prevent potential leakage of tumor cells during and after the surgery, the injection site is sealed with bone wax. This method requires a minor surgery of approximately 15 minutes under anesthesia. Our established orthotopic osteosarcoma model could serve as a valuable and reliable tool for examining tumor progression of various types of bone tumors.
Keywords: osteosarcoma, orthotopic, intrafemoral, mouse model, leak, metastasis
1. Introduction
Osteosarcoma is the most common type of bone cancer in children and young adults. It generally occurs in the long bones, and sixty percent of the cases localize around the knee (40% in the distal femur and 20% in the proximal tibia). At the time of diagnosis, 15–20% of patients with osteosarcoma have clinically detectable metastases. More importantly, almost all patients have pulmonary micro-metastasis, making chemotherapy the first choice of treatment. Due to intensive neoadjuvant and adjuvant chemotherapy, the 5-year survival rate for osteosarcoma has improved to 50~80%; however, for metastatic osteosarcoma it remains at 15% to 40% and has not changed over the last three decades [1–4]. This is mainly due to the poor understanding of mechanisms underlying the malignant properties, such as high tumor initiating potential, metastasis, and drug-resistance, in high grade osteosarcoma.
To understand the mechanisms governing malignant properties of bone tumors and to develop novel therapeutic approaches, there is a need for mouse models that recapitulate the entire physiological process of cancer progression. Osteosarcoma originates from mesenchymal stem cells or their progenitor cells which reside in the bone marrow. Hence, it is ideal to transplant osteosarcoma cells into the bone marrow space. Such orthotopic transplantation of tumor cells allows them to grow in a proper microenvironment, thereby most suited for testing the abilities of osteosarcoma cells to initiate tumor formation and metastasize to other organs. Towards this goal, intratibial injection method was previously established [5]. Although it is simple and used in several publications [6–8], it has several technical pitfalls. The major pitfall is leakage of cells from the injection site. This is because the tibia does not have sufficient space in the medullary cavity and the percutaneous injection method does not allow verifying the absence of leakage. Moreover, reaming the intramedullary space of the tibia is not done, likely due to the curved anatomical feature of this bone. Although the intrafemoral injection method also showed successful tumor establishment in the femur [9, 10], no publication described details of the procedures to minimize the leakage. Leakage from the bone marrow cavity could alter the onset of tumor formation and result in experimental variation or inconsistency. Leakage can be mainly caused by confined intramedullary space and increased intramedullary pressure after tumor cell injections.
To overcome these concerns and improve the orthotopic tumor model of osteosarcoma, we have established a novel method for orthotopic injections. We inject tumor cells into the femur, because mouse femur is relatively straight with mild bowing and has larger medullary cavity than tibia. Tumor cell suspension is injected into the femoral cavity from a hole generated at the intercondylar notch after reaming the intramedullary space. Afterwards, the injection hole is sealed with bone wax. These procedures not only allow investigators to directly observe tumor cell suspension into the femur, but also minimize potential leakage from the injection site during and after surgery. This minor surgery takes approximately 15 minutes. Our method demonstrates successful primary osteosarcoma establishment in the femur and metastases to the liver and lungs. This orthotopic tumor model could be a valuable tool for research of any types of bone sarcoma, such as Ewing sarcoma and pleomorphic sarcoma.
2. Materials and Methods
2.1. Animal maintenance
Non-obese diabetic/severe combined immunodeficiency (NOD-scidIL2Rγnull) mice at 6 weeks old were purchased from Charles River laboratories (Wilmington, MA). Mice were maintained under specific pathogen free conditions, and experimental procedures were performed according to the protocol approved by Institutional Animal Care and Use Committee.
2.2. Cell lines and cell culture
Human osteosarcoma cell lines including SJSA-1 and KHOS/NP were used for intrafemoral injection studies. Cells were maintained in Dulbecco Modified Eagle Medium (DMEM, Corning Cellgro) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 IU/ml penicillin-0.5mg/ml streptomycin (MP Biochemicals, LLC) at 37°C in an atmosphere of 5% CO2 in air.
For the intrafemoral injection studies, osteosarcoma cells were detached using Gibco Cell Dissociation Buffer (Life Technologies) and counted following trypan blue staining. Live cells (up to 1 million) were suspended in 15 µl of 4.5 mg/ml growth factor reduced Matrigel (BD Biosciences) in Hanks balanced salt saline (HBSS) to allow cell suspension to solidify and stay in the femoral cavity, which helps minimize the leakage.
2.3. X-ray imaging
X-ray images were taken with Faxitron LX-60 (Tucson, Arizona), using the conditions of 28 kV, 0.3 mA and 10 seconds of exposure time.
2.4. Hematoxylin and eosin (H&E) staining
Tissues were fixed in 10% buffered formalin overnight and transferred to 70% ethanol. Tissues were then paraffin-embedded, sectioned (5 µm), and used for standard H&E staining [11].
3. Description of intrafemoral injection procedure
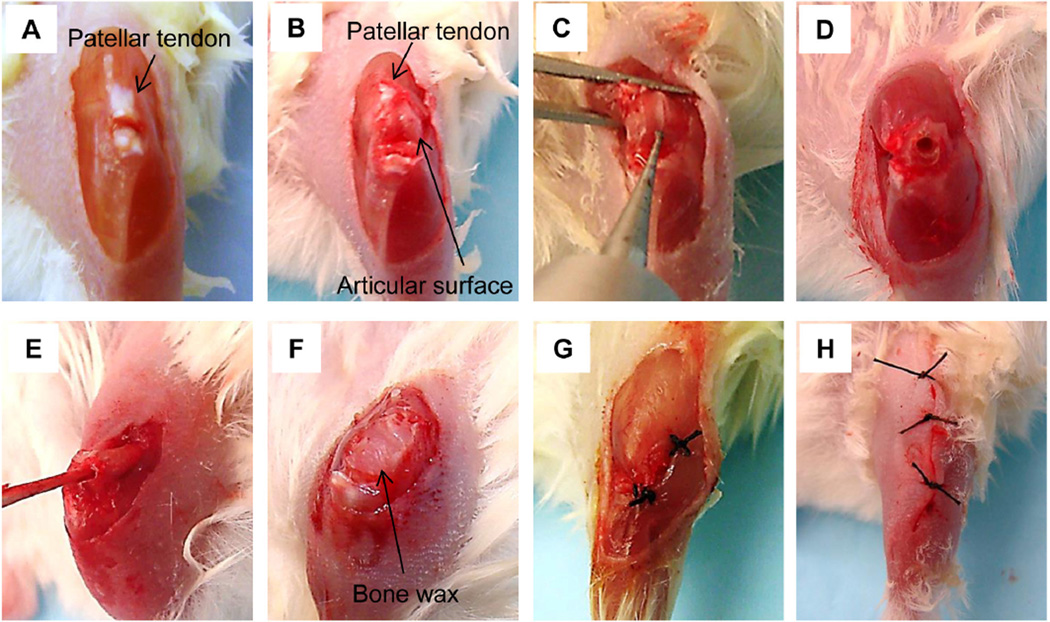
All instruments were sterilized by autoclaving before surgery. Mice were anesthetized with 1–5% isoflurane. The hair around the knee joint of one leg was removed and skin was then scrubbed with iodophor and 70% ethanol. The knee of the mouse was flexed beyond 90 degree and a longitudinal skin incision was made across the front of the left knee (Fig. 1A). The patellar tendon was transected near the tendon attachment site of the tibia and reversed to the proximal side to expose condyles of the distal femur (femoral articular surface, Fig. 1B). The vastus lateralis muscle was separated by a scalpel to expose distal shaft of the femur. In this way, the surface of the femoral articular was exposed to make a hole for tumor cell injection. Using an electric drill (Fine Science Tools Inc Foster city, CA), a small hole was made to reach the bone marrow space (Fig. 1C, D). Then, a small stick was inserted through the hole and was turned around to ream the bone marrow space (Fig. 1E). This procedure helps reduce intramedullary pressure and retain sufficient space for tumor cell injections. To stop the bleeding, small gauzes were inserted into the bone marrow space. After confirming that bleeding had ceased, 15 µl of cell suspension in Matrigel in HBSS was injected into the intrafemoral space using a 27 gauge needle with a tuberculin syringe. Cell numbers can range from as low as 100 cells to 1 million depending on the experiments. Following tumor cell injection into the medullary space of the femur, the hole was immediately sealed with bone wax (World Precision Instrument Inc, Sarasota, FL) to prevent the potential leakage of cell suspension (Fig. 1F). Then, the patellar tendon was sutured near the tendon attachment site of the tibia (Fig. 1G). In case that suturing to the tendon attachment site was not feasible, the patellar tendon was sutured to the tibialis anterior muscle. This procedure did not cause any dysfunction of the mouse leg. Finally, the skin was closed using 5-0 Surgilon (Fig. 1H) and wiped with iodophor and 70% ethanol. Total operation time per one procedure was approximately 15 minutes.
Fig, 1.
Procedure of intrafemoral injection. (A) Skin incision and transection of the patellar tendon. (B) Exposure of the articular surface of the distal femur. (C, D) Drilling the articular surface of the distal femur to generate a hole. (E) Reaming the intramedullary space with a stick. (F) Sealing the injection hole with bone wax. (G) Reconstruction of the patellar tendon. (H) Skin closure.
4. Outcome of intrafemoral injections of osteosarcoma cells
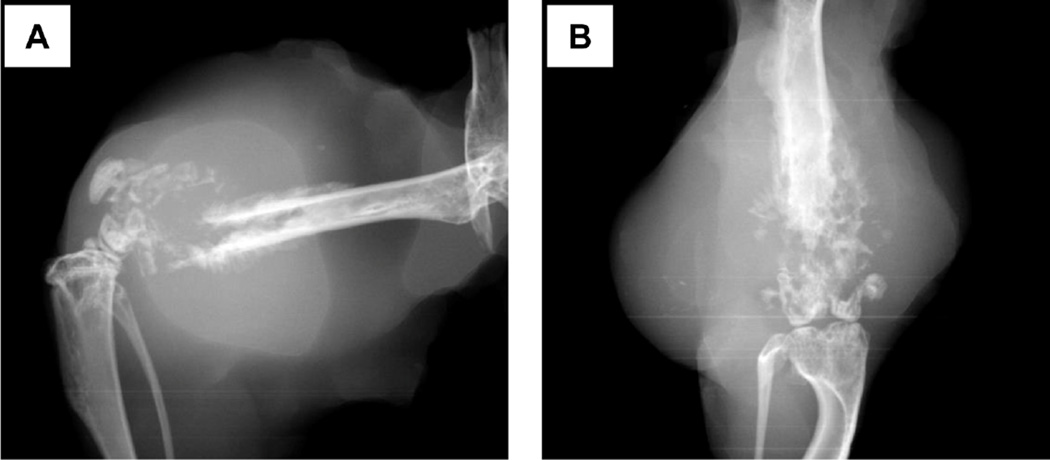
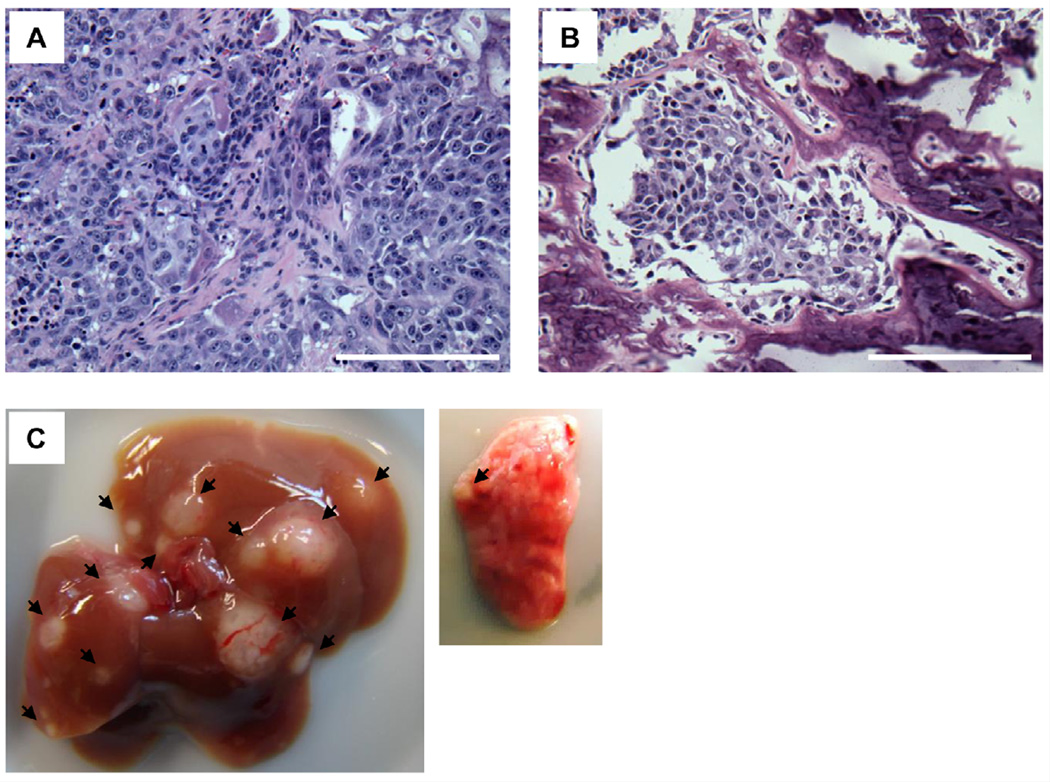
To demonstrate tumor formation, X-ray images were taken one month after KHOS/N P cell injections (1,000,000 cells), showing a soft tissue mass and bone destruction with periosteal reaction and production of an osteoid matrix (Fig. 2A, B). Importantly, the distal end of the femur was nearly intact, indicating that the tumor had arisen from inside of the bone, but not from leakage. Also, histopathological specimen sections were made. H&E stains revealed mitotically active spindle shaped cells, highly pleomorphic malignant cells, osteoid formation with eosinophilic staining, and invading malignant cells into bone cortex (Fig. 3A, B). We also detected metastatic nodules to the liver and lungs (Fig. 3C).
Fig. 2.
Representative X-ray images of a tumor derived from KHOS/NP cells (1,000,000). (A) Lateral view. (B) Frontal view.
Fig. 3.
(A) A representative image of H&E staining of a xenografted tumor derived from KHOS/NP osteosarcoma cells. (B) An image of H&E staining showing invasion of the femur by a KHOS/NP-derived tumor. Scale bar: 200 µm. (C) Images of metastases of a KHOS/NP-derived tumor to the liver (left) and lungs (right). Arrows indicate metastases.
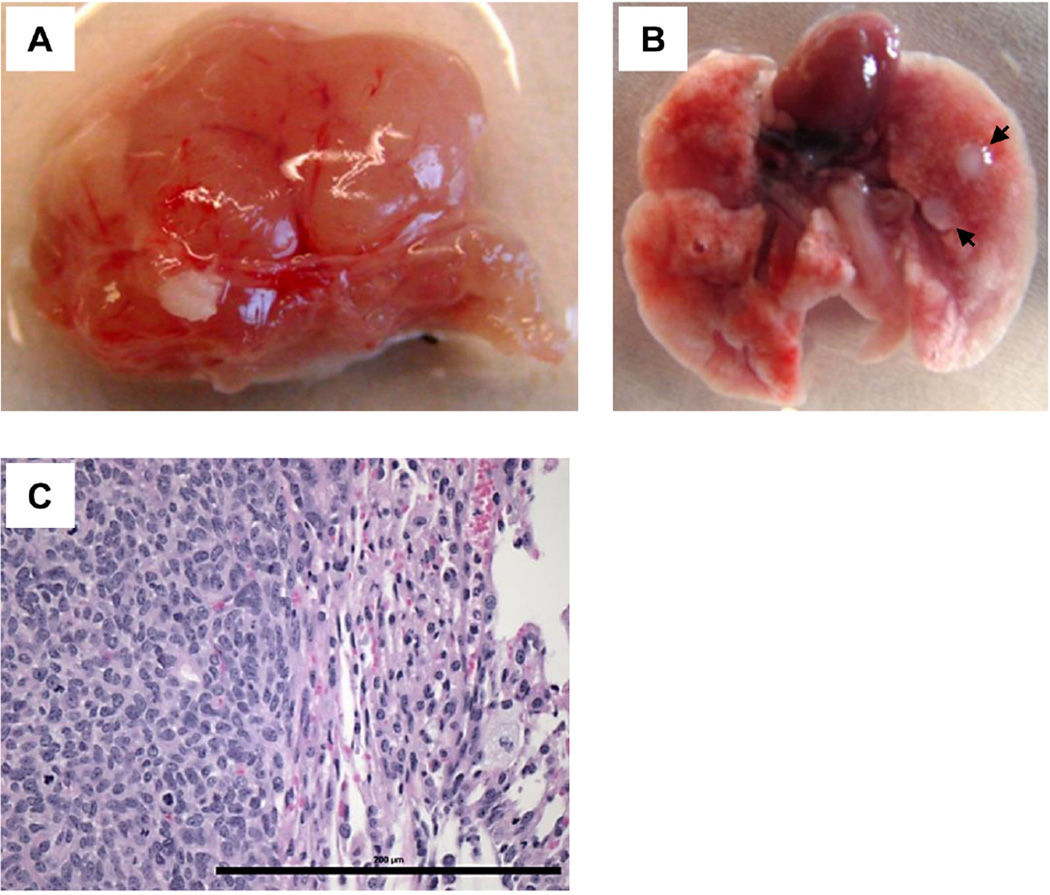
Another set of experiment was also performed using SJSA-1 osteosarcoma cells. Approximately 2–3 months after tumor cell injection (100,000 cells), we detected a primary tumor formation in the femur (Fig. 4A), as well as metastases to the lungs (Fig. 4B) showing malignant mesenchymal cells in the lungs in H&E staining (Fig. 4C).
Fig. 4.
(A) A representative image of a primary tumor following intrafemoral injection of SJSA-1 cells (100,000). (B) An image of lung metastases. Arrows indicate metastases. (C) A representative image of H&E staining of a SJSA-1 lung metastasis. Scale bar: 200 µm.
5. Discussion
Xenograft mouse models of cancer are crucial for elucidating physiological functions of human proteins in cancer and developing novel cancer therapies. In the past few decades, various xenograft animal models have been established including ectopic and orthotopic models [12–15]. In ectopic models, such as subcutaneous injections of bone and soft tissue sarcomas, the transplanted sites are different from the origin of tumors. This is the most frequently used model due to the simplicity of this procedure and feasibility of measurement of tumor growth. However, the major issue with the ectopic model is that tumor cells are placed in an inappropriate microenvironment different from where they arise in nature, because the progression of tumors is dependent on the characters of tumor cells themselves and host factors present in the proper micro-environments. Hence, this model is not suited for examining cancer invasion and metastasis [16, 17]. In this scenario, orthotopic models in which tumor cells are transplanted into the original site of the tumor are physiological and suited for examining tumor progression in vivo. Indeed, metastasis, which is not easily observed following subcutaneous injections, can be observed using an orthotopic model [7]. However, there are some concerns with this method. These include reproducibility of tumor growth due to leakage of injected tumor cells from the injection hole and difficulty of measuring tumor growth. In vivo tumor monitoring could be overcome by labeling cells with fluorescence or luciferase and performing in vivo bio-imaging systems; however, the leakage problem is heavily dependent on the researcher’s skill. Thus, it is important to develop a reliable technique that can be easily followed by any researchers to obtain reproducible results.
For osteosarcoma, Berlin et al. [5] was the first group to perform orthotopic injections of v-Ki-ras-transformed human osteosarcoma cells (KRIB) into intramedullary space of the tibia and demonstrate successful establishment of osteosarcoma and lung metastases in nude mice. This percutaneous injection is simple but does not allow investigators to confirm whether tumor cells indeed stay in the intramedullary space during and after tumor cell injections. Additionally, intrafemoral injection method was previously published [9, 10]. However, details of these methods, including reaming of the intramedullary space and the way to minimize leakage, were not described. The leakage could be the major concern of this method, since it could alter the onset of tumor establishment and cause experimental inconsistency. We therefore modified the existing orthotopic osteosarcoma mouse model and established a method to minimize the leakage. In our method, investigators are required to perform a minor surgery under anesthesia. The major improvements are to drill the intercondylar notch of the femur and to make a hole for reaming the intramedullary space and reducing intramedullary pressure. In this way, investigators can directly observe tumor suspension to be injected into the femoral cavity and detect if any leakage occurs. Moreover, following injections, bone wax is used to seal the injection hole to prevent any leakage during and after surgery.
Our established orthotopic injection method with minimized leakage is easy to perform and hence can provide with reproducible and consistent results. This method can be used for not only osteosarcoma, but also for other malignant bone-originated tumors, such as pleomorphic sarcoma and Ewing sarcoma. Additionally, this method can be used as a bone metastasis model, including breast and prostate cancer. Indeed, intratibial injection is used for metastatic models of breast and prostate cancer [18–23]. Moreover, it is extremely useful for cancer stem cell research of bone tumors, where orthotopic injections of small number of cancer cells are required to demonstrate tumor initiating potential of cellular populations having stem-like properties, since establishment of tumors is dependent on the number of tumor initiating cells within cells transplanted [24]. Furthermore, several reports demonstrate that co-injection of human cancer cells with human stromal cells enhances tumor growth, suggesting the significance of proper tumor microenvironment [8, 25]. It would be interesting to compare tumor growth between injection of human osteosarcoma cells alone and co-injection of osteosarcoma cells with human stromal cells, using our established model as a future study. Nonetheless, we believe that mouse bone marrow provides better microenvironment for human osteosarcoma cells than subcutaneous injections, since the orthotopic injection of osteosarcoma cells facilitates spontaneous metastasis which is uncommon following the subcutaneous injections.
Our established method can also be used for patient-derived tumor xenografts (PDTX) [26, 27]. Instead of injecting patient’s tumors subcutaneously, placing them in proper environments may improve tumor forming efficacy and allow original genetic and pathological features to be maintained. Since PDTX is useful for developing new anticancer drugs and personalized cancer therapy, our developed intrafemoral injection method may contribute to accelerating the development of novel treatment strategies for cancers occurring in the bone.
Acknowledgments
We thank Amit S. Adhikari, Neeraj Agarwal, Alejandro B. Parrales, Atul Ranjan, and Marsha Danley for helpful discussion and technical assistance. We thank Dr. Yasuyoshi Ueki and Dr. Mizuho Kittaka for taking X-ray images. This project is supported by NIH 1- R01-CA174735-01A1 (T.I.), 8-P30-GM103495 (B.T.), and 5-P30-CA168524-02 (T.I., D.R.W.) grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whelan JS. Osteosarcoma. European journal of cancer. 1997;33:1611–1618. doi: 10.1016/s0959-8049(97)00251-7. discussion 1618–1619. [DOI] [PubMed] [Google Scholar]
- 2.Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer metastasis reviews. 2009;28:247–263. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 3.Stiller CA, Craft AW, Corazziari I E.W. Group. Survival of children with bone sarcoma in Europe since 1978: results from the EUROCARE study. European journal of cancer. 2001;37:760–766. doi: 10.1016/s0959-8049(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 4.Sergi C, Zwerschke W. Osteogenic sarcoma (osteosarcoma) in the elderly: tumor delineation and predisposing conditions. Experimental gerontology. 2008;43:1039–1043. doi: 10.1016/j.exger.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Berlin O, Samid D, Donthineni-Rao R, Akeson W, Amiel D, Woods VL., Jr Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer research. 1993;53:4890–4895. [PubMed] [Google Scholar]
- 6.Yuan J, Ossendorf C, Szatkowski JP, Bronk JT, Maran A, Yaszemski M, Bolander ME, Sarkar G, Fuchs B. Osteoblastic and osteolytic human osteosarcomas can be studied with a new xenograft mouse model producing spontaneous metastases. Cancer investigation. 2009;27:435–442. doi: 10.1080/07357900802491477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luu HH, Kang Q, Park JK, Si W, Luo Q, Jiang W, Yin H, Montag AG, Simon MA, Peabody TD, Haydon RC, Rinker-Schaeffer CW, He TC. An orthotopic 13 model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis. 2005;22:319–329. doi: 10.1007/s10585-005-0365-9. [DOI] [PubMed] [Google Scholar]
- 8.Bian ZY, Fan QM, Li G, Xu WT, Tang TT. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101:2554–2560. doi: 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherrier B, Gouin F, Heymann MF, Thiery JP, Redini F, Heymann D, Duteille F. A new experimental rat model of osteosarcoma established by intrafemoral tumor cell inoculation, useful for biology and therapy investigations. Tumour Biol. 2005;26:121–130. doi: 10.1159/000086483. [DOI] [PubMed] [Google Scholar]
- 10.Raheem O, Kulidjian AA, Wu C, Jeong YB, Yamaguchi T, Smith KM, Goff D, Leu H, Morris SR, Cacalano NA, Masuda K, Jamieson CH, Kane CJ, Jamieson CA. A novel patient-derived intra-femoral xenograft model of bone metastatic prostate cancer that recapitulates mixed osteolytic and osteoblastic lesions. Journal of translational medicine. 2011;9:185. doi: 10.1186/1479-5876-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi Q, Ranjan A, Fan R, Agarwal N, Welch DR, Weinman SA, Ding J, Iwakuma T. MTBP inhibits migration and metastasis of hepatocellular carcinoma. Clin Exp Metastasis. 2015;32:301–311. doi: 10.1007/s10585-015-9706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook N, Jodrell DI, Tuveson DA. Predictive in vivo animal models and translation to clinical trials. Drug discovery today. 2012;17:253–260. doi: 10.1016/j.drudis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Jung J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicological research. 2014;30:1–5. doi: 10.5487/TR.2014.30.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:971–981. [PubMed] [Google Scholar]
- 15.Firestone B. The challenge of selecting the 'right' in vivo oncology pharmacology model. Current opinion in pharmacology. 2010;10:391–396. doi: 10.1016/j.coph.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 17.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutation research. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AS, Figg WD. In vivo models of prostate cancer metastasis to bone. The Journal of urology. 2005;174:820–826. doi: 10.1097/01.ju.0000169133.82167.aa. [DOI] [PubMed] [Google Scholar]
- 19.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. International journal of cancer. Journal international du cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, Vessella RL. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:295–306. [PubMed] [Google Scholar]
- 21.Corey E, Quinn JE, Bladou F, Brown LG, Roudier MP, Brown JM, Buhler KR, Vessella RL. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. The Prostate. 2002;52:20–33. doi: 10.1002/pros.10091. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Zhou H, Fong-Yee C, Modzelewski JR, Seibel MJ, Dunstan CR. Bone resorption increases tumour growth in a mouse model of osteosclerotic breast cancer metastasis. Clinical & experimental metastasis. 2008;25:559–567. doi: 10.1007/s10585-008-9172-4. [DOI] [PubMed] [Google Scholar]
- 23.Lev DC, Kim SJ, Onn A, Stone V, Nam DH, Yazici S, Fidler IJ, Price JE. Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:306–314. [PubMed] [Google Scholar]
- 24.Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T. CD117 and Stro-1 Identify Osteosarcoma Tumor-Initiating Cells Associated with Metastasis and Drug Resistance. Cancer research. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto S, Honoki K, Fujii H, Tohma Y, Kido A, Mori T, Tsujiuchi T, Tanaka Y. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int J Oncol. 2012;40:163–169. doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 26.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nature reviews. Clinical oncology. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer research. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]