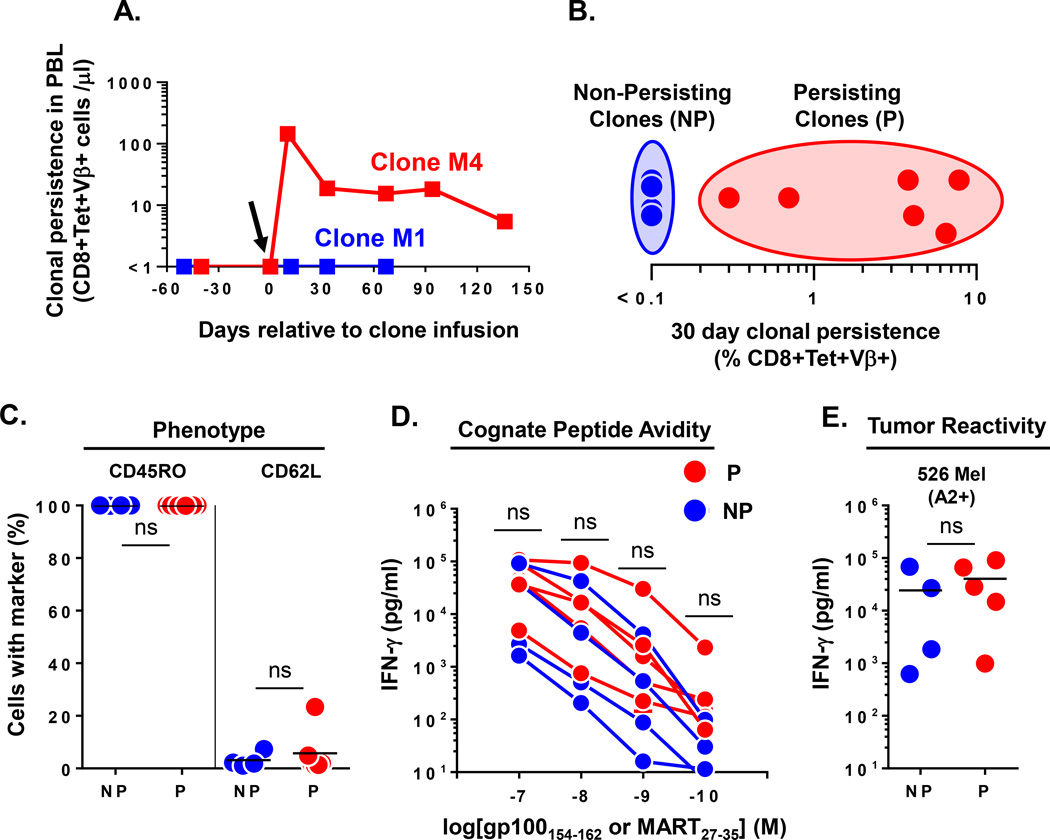
Figure 1. Heterogeneous engraftment of CD8+ T effector clones after adoptive transfer in humans.
(A.) Dichotomous long term engraftment of M1 and M4 MART specific CD8+ T effector clones as determined by the absolute number of CD8+Tetramer+Vβ+ T cells per microliter of blood at time points relative to infusion in respective melanoma patients. Arrow denotes the day of clone infusion. (B.) Heterogeneous persistence of infused effector clones (n=10 clones infused into 9 patients) as assessed by % CD8+Tetramer+Vβ+ cells identified in PBMC at day 30 post infusion. Non-persisting (NP) clones were defined to have engraftment levels of ≤0.1% of CD8+ T cells; Persisting (P) clones had engraftment levels >0.1% of CD8+ T cells. (C.) Pre-infusion phenotype of NP (n=4) and P (n=6) effector clones as determined by FACS expression of the indicated differentiation markers (% of cells). (D.) Cognate peptide avidity and (E.) reactivity against 526 Mel (A2+) tumor cell line for NP (n=4) and P (n=5) effector clones. Shown are supernatant IFN-γ levels after subtracting background reactivity against either control peptide or 888 Mel (A2−) tumor cell line. Statistical comparison performed by unpaired T test; ns, non-significant. Bar on graphs represents mean.