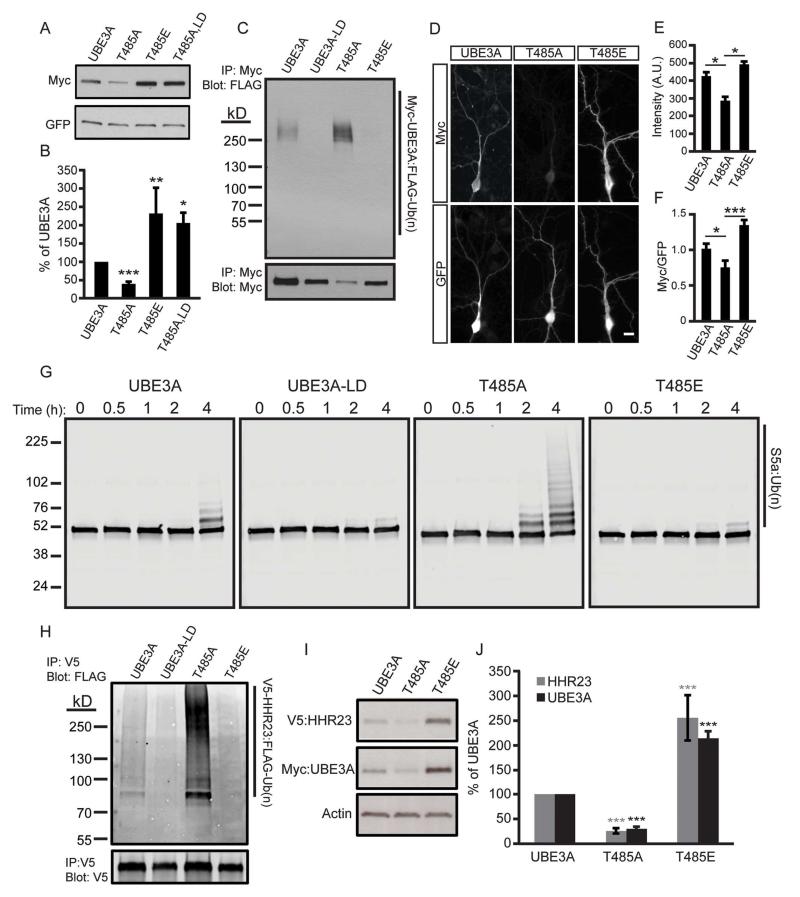
Figure 4. Phosphorylation at T485 inhibits UBE3A ubiquitin ligase activity.
(A and B) Representative western blot and quantification of HEK293T cells transfected with the indicated constructs. Values are shown as the percent of WT UBE3A levels ± standard error, n=4, *p<0.05, **p<0.005, ***p<0.0005.
(C) HEK293T cells transfected with the indicated Myc-UBE3A and FLAG-ubiquitin constructs were treated with the proteasome inhibitor MG-132 (30 μM, 4 h). UBE3A was immunoprecipitated using an anti-Myc antibody, and western blot probed with an anti-FLAG antibody to detect ubiquitinated UBE3A.
(D - F) Immunofluorescence staining and quantification of WT UBE3A and T485 mutants in DIV 10 mouse cortical neurons, scale bar 15 μm. Raw intensity values for Myc immunofluorescence (E) or Myc immunofluorescence normalized to GFP (F) are shown as the mean intensity ± standard error. T485A, n=14; T485E, n=16, *p<0.05, ***p<0.0005.
(G) In vitro ubiquitination assay was performed using S5a as the substrate and UBE3A mutants expressed and purified from HEK293T cells. Reactions were stopped after 4 h and the formation of high molecular weight polyubiquitinated S5a was monitored by western blot using an S5a antibody.
(H) HEK293T cells were transfected with the indicated Myc-UBE3A, FLAG-ubiquitin, and V5-HHR23A constructs and treated with MG-132. HHR23A was immunoprecipitated and western blot probed with an anti-FLAG antibody.
(I and J) Western blot and quantification of protein lysates from HEK293T cells transfected with the indicated constructs. Values are expressed as the mean percent ± standard error of protein levels in WT UBE3A expressing cells, n=4, ***p<0.0005.