Abstract
The first objectives of this article are to review the structure, chemistry, and physiology of bile acids and the types of bile acid malabsorption observed in clinical practice. The second major theme addresses the classical or known properties of bile acids, such as the role of bile acid sequestration in the treatment of hyperlipidemia; the use of ursodeoxycholic acid in therapeutics, from traditional oriental medicine to being, until recently, the drug of choice in cholestatic liver diseases; and the potential for normalizing diverse bowel dysfunctions in irritable bowel syndrome, either by sequestering intraluminal bile acids for diarrhea or by delivering more bile acids to the colon to relieve constipation. The final objective addresses novel concepts and therapeutic opportunities such as the interaction of bile acids and the microbiome to control colonic infections, as in Clostridium difficile-associated colitis, and bile acid targeting of the farnesoid X receptor and G protein-coupled bile acid receptor 1 with consequent effects on energy expenditure, fat metabolism, and glycemic control.
Keywords: bile acids, pharmacotherapeutics
bile acids (BAs) are steroid-derived detergent molecules that are excreted by the liver and are responsible for fat emulsification in the small intestine, aiding fat digestion and absorption. There are several BA species that are differentiated structurally by their hydroxylation and conjugation status. BAs have been used as medicinals for several centuries, and the known properties of BAs as a shunt pathway for lipids led to the use of BA sequestrants in the treatment of hyperlipidemia. Relative to the deleterious hydrophobic BAs, the use of ursodeoxycholic acid (UDCA) led to their extensive use as the drug of choice in cholestatic liver diseases, though recent advances suggest that it may be replaced in the future by farnesoid X receptor (FXR) agonists or fibroblast growth factor 19 (FGF-19) analogs, both of which have the capacity to reduce the hepatocyte synthesis of BAs. The intraluminal effects of relatively high concentrations of di-α-hydroxy BA [though not at lower concentrations, which may be antisecretory (27)] result in diarrhea, and, conversely, neutralization of the effects of such BAs results in relief of BA diarrhea. Recent research has ushered in other therapeutic opportunities such as interaction with the microbiome and effects on energy expenditure, fat metabolism, and glycemic control. This review addresses the biochemical, pathophysiological, and mechanistic concepts associated with the use of BAs in therapeutics.
Synthesis of Bile Acids from Cholesterol
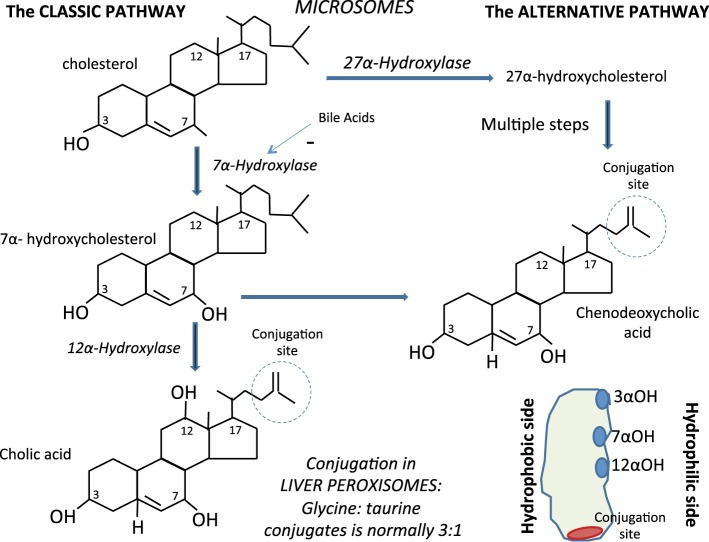
Chenodeoxycholic acid (CDCA) and cholic acid (CA) are the primary BAs that are synthesized in the liver from cholesterol (Fig. 1). In microsomes, cholesterol is converted by 7α-hydroxylase (CYP7A1) to 7α-hydroxycholesterol. Through a series of steps involving NADPH, coenzyme A (CoA), chenodeoxycholyl-CoA and cholyl-CoA are formed; formation of cholyl-CoA requires an additional hydroxyl group, catalyzed by the enzyme 12α-hydroxylase. Finally, these molecules are conjugated with the amino acids glycine or taurine in peroxisomes. The ratio of the glycine to the taurine conjugates is normally 3:1. In humans, an alternative BA synthetic pathway also exists, producing less than 10% of the total BAs. In this pathway, the mitochondrial sterol 27-hydroxylase (CYP27A1) generates 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid from cholesterol, and these intermediates feed into a pathway leading to the formation of CDCA (30).
Fig. 1.
Synthesis of primary bile acids from cholesterol in hepatic microsomes. The main primary bile acids, cholic acid and chenodeoxycholic acid, are synthesized in hepatocyte microsomes via classic or alternative pathways; the bile acids are then conjugated with glycine or taurine in liver peroxisomes to produce the amphipathic structure that contains both hydrophobic (lipid-soluble) and polar (hydrophilic) regions. The cholesterol portion of a bile acid is hydrophobic and the amino acid conjugate is polar and hydrophilic.
Chemical Structures of Bile Salts
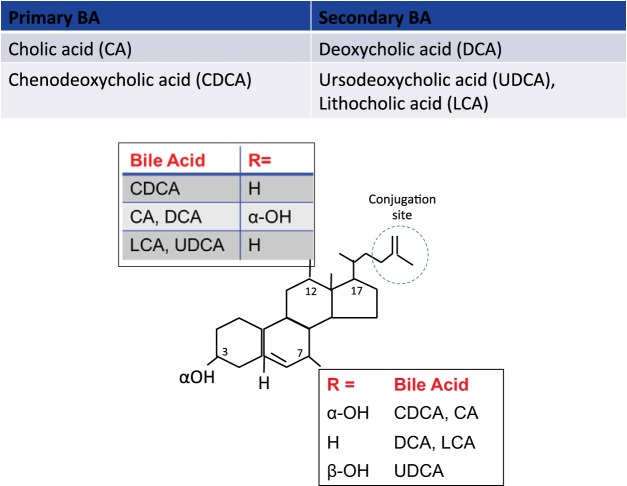
The chemistry of BAs is characterized by one (lithocholic acid, LCA), two (CDCA; and deoxycholic acid, DCA), or three (cholic acid, CA) hydroxyl groups in α configuration. Another BA (which constitutes <5% of the BA pool) is UDCA, in which there is a hydroxyl group in an α configuration at the third carbon atom and a hydroxyl group in the β configuration at the seventh carbon atom (Fig. 2). The hydroxyl groups are located on the hydrophilic side of the BA molecule (Fig. 1). Deconjugation (by bile salt hydrolase, which is commonly produced by the normal microbiota) and dehydroxylation of primary BAs in the colon result in the production of LCA and DCA (Fig. 2).
Fig. 2.
Structure of common primary and secondary bile acids. The most prevalent bile acids in humans are tri-hydroxy cholic acid (CA), di-hydroxy chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), and the mono-hydroxylated lithocholic acid (LCA). The figure shows the differences in structure based on the presence of H− or OH− at the carbon 3, 7, and 12 positions. In UDCA, the OH− group at the C7 position is in the β configuration, in contrast to CDCA (OH− at C7 in α configuration). Dehydroxylation results from bacterial metabolism, predominantly in the colon. R, atom or group attached to rest of the molecule.
The chemistry of BAs determines their biological effects. For example, two hydroxyl groups [3,7 or 3,12] in α configuration are associated with the increased fluid secretion by CDCA and DCA, respectively, in the colon. In contrast, UDCA (with 3α, 7β configuration) does not induce fluid secretion, and it reduces secretion caused by activators of adenyl cyclase, such as forskolin, (28).
Another example illustrating the structural specificity of actions of BAs is the induction of apoptosis in colonic epithelial cells (5) and hepatocytes (55). Thus, whereas CDCA, LCA, and DCA induce apoptosis, UDCA prevents apoptosis.
Enterohepatic Circulation of Bile Acids
BAs are excreted from the liver as conjugates to the amino acids taurine or glycine, and they are concentrated in the gall bladder before being released into the duodenum, typically in response to ingestion of a meal or concurrent with an interdigestive migrating motor complex in the neighboring stomach and proximal small intestine (48). BAs remain ionized in the duodenum. Ionization increases their solubility, allowing a sufficiently high concentration to achieve spontaneous formation of micelles, whereby the polar BAs surround fat molecules and can present the hydrophobic fat molecules (which are insoluble in the aqueous phase) to the brush border membrane of the small intestine for digestion and absorption.
The apical Na+-dependent bile salt transporter (ASBT) [also called ileal BA transporter (IBAT) or SLC10A2 (solute carrier family 10, member 2)] is responsible for the active reuptake of BAs in the terminal ileum to enter the portal circulation, reabsorbing ∼95% of BAs and transporting them to the liver. BAs are agonists of the FXR, the nuclear receptors found in epithelial cells including ileal enterocytes and hepatocytes. The level of enterocyte BA leads to the production of the endocrine factor, FGF-19, which is released from ileal enterocytes into the portal circulation and activates FGF receptor 4 (FGF-R4) on the hepatocyte membrane in a process that involves interaction with klothoβ. This activation downregulates CYP7A1, inhibiting BA synthesis. This enterohepatic circulation of BAs occurs about five times per day.
The 5% BA pool that is not absorbed in the ileum undergoes deconjugation and dehydroxylation by colonic bacteria BAs to produce secondary BAs, DCA, and LCA (22). Disorders of the enterohepatic circulation result in BA malabsorption.
Bile Acid Malabsorption
There are three classes of BA malabsorption. Type 1, also called ileal dysfunction and impaired reabsorption, is found in Crohn's disease. Type 2, also called idiopathic BA diarrhea, produces increased fecal BAs, watery diarrhea, and response to BA sequestrants in the absence of ileal or other obvious gastrointestinal disease. Type 3 is found in other gastrointestinal disorders that affect absorption such as small intestinal bacterial overgrowth, celiac disease, or chronic pancreatitis (19). Recent studies have proposed mechanisms to explain idiopathic BA diarrhea: first, defective feedback inhibition of hepatic synthesis caused by reduced production of FGF-19 by ileal enterocytes (53); second, genetic variations reducing the levels of hepatic proteins involved in feedback regulation of BA synthesis (klothoB and FGFR4 genes) (11, 56); third, a gain-of-function variation in the gene that results in upregulation of the membrane bound BA receptor, TGR5 or G protein-coupled BA receptor 1 (GPBAR1) (12).
Current and Potential Therapeutic Applications of Bile Acid Modulation
Bile acid sequestration for hyperlipidemia.
BAs provide a natural excretory pathway for hepatic cholesterol. BA sequestrants (e.g., cholestyramine, colestipol, colesevelam) bind negatively charged BAs and bile salts in the small intestine and interrupt the enterohepatic circulation of BAs, leading to increased conversion of cholesterol into bile within the liver. These effects result in decreased hepatocyte cholesterol content, promoting an increase in LDL receptors and increasing the clearance of LDL from the circulation (24). BA sequestrants that lower LDL cholesterol result in up to 10% increase in HDL (“good”) cholesterol; unfortunately, they also increase serum triglyceride levels. Thus BA sequestrants are most useful in patients with high LDL cholesterol levels as the major lipid abnormality. BA sequestrants can be combined with statins or niacin to achieve greater reduction in LDL cholesterol, and they tend to be used alone as initial therapy with elevated LDL cholesterol in patients at low risk, or in those intolerant of statins. There are problems with BA sequestrants, including poor compliance with treatment with the resins, interference with the absorption of concomitant medications, aggravation of severe constipation, and contraindication in patients with possible bowel or total biliary obstruction (24).
Ursodeoxycholic acid: from traditional oriental medicine to treatment of cholestatic liver diseases.
Bile from 44 different animals (both invertebrates and vertebrates, including human bile) has been used for centuries for a host of maladies in traditional Chinese medicine beginning in the Zhou dynasty (∼1046-256 BCE) (54). The principal chemicals in animal bile are steroidal detergent-like molecules, bile salts, bile pigment (bilirubin and its glucuronides), membrane lipids, vitamins A, D, E, and K, and melatonin. Bile was claimed to improve liver function, dissolve gallstones, inhibit bacterial and viral multiplication, and promote cardiac chronotropism (regularity of heart rhythm) and was used as an anti-inflammatory, antipyretic, antioxidant, sedative, anticonvulsive, antiallergenic, anticongestive, antidiabetic, and antispasmodic. Pig, wild boar, and human bile diluted with alcohol were shown to form an artificial skin for burns and wounds one thousand years ago in the Tang dynasty (618–907 CE).
In Japan, UDCA was brought to market in the 1950s by Tokyo Tanabe as a liver tonic, based on the legendary therapeutic effects of bear bile. It was packaged as 10 mg tablets. The recommended dose likely had no pharmacodynamic effects (23).
UDCA is the drug of first choice in the treatment of cholestatic liver diseases, the most common being primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) (26). PBC is characterized by interlobular duct destruction, female preponderance (10 F:1 M), autoimmunity with antimitochondrial antibodies in 95% of patients, and several known T lymphocyte targets. In contrast, PSC is characterized by scarring and irregularity of the caliber of intra- and extrahepatic ducts, is more common in males (2 M:1 F), and is associated with ulcerative colitis and unknown T lymphocyte targets (26, 51). UDCA counteracts the cholestatic components in PBC and PSC and exhibits anti-inflammatory and immunomodulatory properties.
The beneficial effects of treatment with UDCA include 1) protection against cytotoxicity due to more toxic BAs; 2) stimulation of hepatobiliary secretion with increased hydrophilicity index of the circulating BA pool, antioxidant activity (due in part to an enhancement in glutathione levels); 3) cytoprotection against BAs and cytokine-induced injury; 4) immunosuppressive properties by interfering with B and T cell functions; 5) inhibition of liver cell apoptosis through targeting of mitochondrial function and integrity, reduction of endoplasmic reticulum stress, and interactions with survival signals in the cAMP, Akt, nuclear factor kappa-B (NF-κB), mitogen-activated protein kinases (MAPK), and phosphatidylinositol 3-kinase (PI3K) signaling pathways (39, 41).
In PBC, normalization of biochemical values occurs within 2 years in 20% of patients and in an additional 15% of patients after 5 years. Although long-term, high-dose UDCA therapy is associated with improvement in serum liver tests in PSC, it unfortunately does not improve survival and appears to be associated with higher rates of serious adverse events such as mortality, need for liver transplantation, and development of esophageal or gastric varices (31, 32). In fact, meta-analyses show that UDCA does not significantly impact the mortality or need for transplantation in either PBC or PSC (20, 51).
Bile acid “push or pull” for bowel dysfunctions in irritable bowel syndrome.
BAs have been used as therapy for gallstones and cholestatic liver disease since the early 1970s. CDCA was associated with development of diarrhea. In gallstone patients with chronic constipation, CDCA increased stool frequency and loosened stool consistency compared with placebo (6). Some BAs are essentially physiological laxatives. On the other hand, UDCA was associated with no diarrhea. These clinical observations were consistent with the physiological studies performed in animal models and in vitro (13, 28). Fluid secretion occurs through a number of mechanisms, including stimulation of intracellular messengers such as cAMP and calcium, which results in activation of chloride secretion through the cystic fibrosis transmembrane regulator (2, 3, 38). Apart from inducing colonic secretion, BAs induce high-amplitude propagated contractions of the colon (4). These effects are, at least partly, mediated through TGR5, which has been identified in enteric cholinergic and nitrergic neurons and smooth muscle (40) and is associated with the prokinetic actions of BAs (1).
In healthy volunteers and patients with irritable bowel syndrome constipation, CDCA accelerates colonic transit, increases stool frequency, loosens stool consistency, and eases passage of stool (37, 42). Similar effects were observed with an IBAT inhibitor, elobixibat, in chronic idiopathic constipation (14, 57).
BA sequestrants have been the mainstay of treatment of BA diarrhea or malabsorption, whether associated with ileal disease or resections of >100 cm, and of functional diarrheas (10, 17, 37, 49) resulting from one of several candidate mechanisms including deficiency of the ileal hormone FGF-19 (53), variations in genes involved in BA synthesis, feedback regulation, or TGR5 (9, 12).
Bile Acids and the Microbiome
BA pool size is a function of microbial metabolism of BAs in the intestines. The host and microbiome regulate the BA pool size. Thus there are differences between germ-free and conventional mammals; there is higher synthesis of BAs in germ-free than in conventional mice; in the germ-free animals, the primary BA, tauro-β-muricholic acid, inhibits FXR, reducing FGF-15 (the mouse homolog of FGF-19) in the portal circulation and, thereby, enhancing hepatocyte BA synthesis (for review, see Ref. 43).
The host produces a conjugated hydrophilic BA pool, maintained by feedback antagonism of FXR in the intestine and liver. The presence of intestinal microbial flora results in deconjugation and dehydroxylation of the primary BAs (tauro-β-muricholic acid) to produce secondary BAs (β-MCA and hyodeoxycholic acid in mice), which then activate FXR in ileal enterocyte to produce FGF-15. FGF-15 reaches the liver via the portal circulation and reduces hepatocyte BA synthesis, by inhibiting CYP7A1, the rate-limiting enzyme in BA synthesis, which is under the control of FXR. Thus a dynamic equilibrium exists between diet, gut microbiome, and BA pool size/composition.
Despite having a greatly reduced BA pool size (decreased ∼71%) compared with germ-free mice, conventional mice have significantly higher levels of BAs in the cecum (45), possibly because they have downregulation of ASBT, which is under control of intestinal FXR. In contrast to the germ-free mice in which tauro-β-muricholic acid antagonizes FXR, conventional mice have higher FXR available to downregulate the ASBT, and hence more BAs pass into the cecum.
BAs have both direct antimicrobial effects on gut microbes and indirect effects through FXR-induced antimicrobial peptides (7, 25). When rats are fed BAs, there are complex changes in the gut microbiome such as large expansion of Firmicutes and the Clostridia class, with expansions at the genus Blautia, which includes many species of Clostridium and Ruminococcus. In general, expansion of Firmicutes results in greater production of secondary BAs such as DCA.
Presence of the microbiome alters the composition of the BA pool, which in turn has a profound effect on the BA pool size; however, dietary and antimicrobial compounds can affect this equilibrium. Perturbations in this equilibrium can result in pathological states. An intriguing example is the combination of the effects of diet (milk or polyunsaturated fat) and a host factor (taurine-conjugated BAs), leading to an expansion of a pathobiont (Bilophila wadsworthia) and resulting in colitis in a genetically predisposed animal model, IL10−/− mice (15).
Diet, antibiotic therapy, and disease states affect the balance of the microbiome BA pool. Bile salts may have an impact on Clostridium difficile infection pathogenesis (50). Thus “precision” microbiome reconstitution is able to restore BA-mediated resistance to Clostridium difficile. Antibiotics can perturb the intestinal microflora, resulting in failure of BA dehydroxylation necessary for the formation of secondary BAs, which normally inhibit the vegetative growth of Clostridium difficile, a precursor to the production of the toxin that produces colitis. Restoration of normal microflora facilitates the production of the secondary BAs, which prevent toxin elaboration by the vegetative growth from the spores of Clostridium difficile. Clostridium scindens, a BA 7α-dehydroxylating intestinal bacterium, is associated with resistance to Clostridium difficile infection and, upon administration, enhances resistance to infection in a secondary BA-dependent fashion (8).
Effects on Metabolism Through FXR and TGR5 Bile Acid Receptors
Enteroendocrine cells in the epithelial layer of the gut are involved in chemosensing; thus there is a rapid increase in circulating glucagon-like peptide (GLP)-1 and GLP-2 (which are produced by intestinal L cells in the ileocolonic mucosa) after a meal, suggesting that humoral signals from the proximal bowel activate L-cell secretion before direct nutrient exposure in the distal intestine. The factors mediating this rapid activation of release of GLP-1 and GLP-2 are glucose-stimulated insulinotropic peptide (GIP) released from enteroendocrine K cells in the duodenum, and neural reflexes involving gastrin-releasing peptide (also called bombesin).
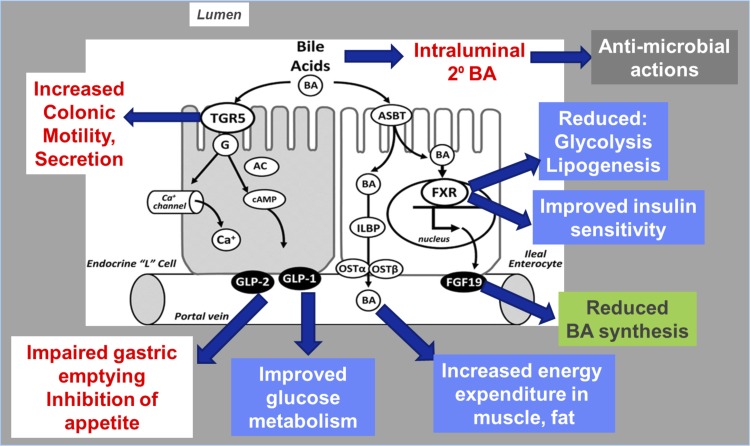
BAs are also signaling molecules (Fig. 3) that induce gut hormone secretion from enteroendocrine cells, such as secretion of GLP-1 through activation of the TGR5, resulting in part of the metabolic “bonus” related to the effects of BAs. BAs have been implicated in metabolic regulation, through FXR-mediated regulation of energy substrate mobilization and storage in the liver. It is intriguing that, in addition to BAs themselves inducing these metabolic effects, the BA sequestrants colesevelam and colestimide have similar metabolic effects. Thus colesevelam lowers glucose and lipid levels in Type 2 diabetes, and colestimide increases secretion of GLP-1 in patients with Type 2 diabetes (reviewed in Ref. 18). The mechanisms mediating the glucose-lowering effect of BA sequestrants remain a matter of controversy (46). Nevertheless, FXR is thought to play a pivotal role in the improved metabolism and weight loss after bariatric surgery, particularly sleeve gastrectomy (44), which is associated with elevated plasma BA levels (35, 47). The beneficial effects of bariatric surgery include metabolic actions via FXR (inhibition of glycolysis and lipogenesis) and TGR5 (increased energy expenditure and improved glucose metabolism) and GLP-1-induced stimulation of insulin secretion and retardation of gastric emptying, contributing to the inhibition of appetite (reviewed in Ref. 29).
Fig. 3.
Summary of actions of bile acids (BA) mediated by farnesoid X receptor (FXR) and TGR5 (G protein-coupled receptor). Bile acids are actively transported into ileal enterocytes by ASBT [apical sodium-coupled bile acid transporter, also known as the ileal bile acid transporter (IBAT)]. Bile acids are agonists at the nuclear FXR activating the synthesis of the hormone, fibroblast growth factor-19 (FGF-19), which has multiple effects including reduced glycolysis and lipogenesis, improved insulin sensitivity, and reduced bile acid synthesis. Independently of FXR receptor actions, the absorbed bile acids increase energy expenditure in muscle and fat. Bile acids also bind the G protein-coupled bile acid receptor TGR5 (triglyceride receptor 5) on enteroendocrine L cells to activate release of glucagon-like peptides (GLP)-1 and -2, which results in retardation of gastric emptying, inhibition of appetite, and improved glucose metabolism. Other TGR5 receptors on intrinsic nerves result in effects of bile acids to increase colonic fluid and electrolyte secretion and motility. G, G protein; AC, adenylate cyclase; ILBP, ileal lipid binding protein; OST, organic solute transporter.
A poorly absorbed FXR agonist termed fexaramine has also been developed that selectively activates intestinal FXR (16). In mice, this FXR agonist potently induces FGF-15, which induces the transdifferentiation of white adipose tissue to brown adipose tissue. The browning of the adipose tissue results in substantial metabolic improvements in mice fed an otherwise obesity-inducing diet.
Dual activation of the BA nuclear receptors, FXR and TGR5 (with INT-767), protects mice against atherosclerosis (33), consistent with the fact that BA agonists of these two receptors have the potential to alter several aspects of glucose and fat metabolism (Fig. 3).
In summary, BAs are involved in the regulation of energy and glucose and lipid metabolism via FXR- and TGR5-mediated signaling pathways.
Applications of an FXR Agonist, Obeticholic Acid, in Human Gastrointestinal and Liver Diseases
Obeticholic acid (or 6-ethyl CDCA) is an FXR agonist that is ∼100 times more potent than naturally occurring BAs (58). When administered to patients with BA diarrhea, the frequency and consistency of bowel movements are reduced (52). Obeticholic acid increased median fasting FGF-19 and significantly reduced hepatocyte synthesis (fasting C4) of BAs, thereby improving stool frequency and consistency (52).
Efficacy of obeticholic acid was tested in PBC and inadequate response to UDCA on alkaline phosphatase in a randomized, controlled trial and open extension (21): biochemical parameters were improved, but the patients developed worse pruritus through an unclear mechanism.
Effects of obeticholic acid in nonalcoholic steatohepatitis showed improvement of several parameters (serum alanine aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase) relative to baseline, including improvements in fibrosis, hepatocellular ballooning, steatosis, and lobular inflation (36). The mechanism is thought to involve FXR activation, decreasing hepatic lipogenesis by downregulation of the transcription factor SREBP (sucrose responsive element binding protein)1c and increasing SIRT (sirtuin)1.
Moreover, a phase 2, double-blind, placebo-controlled, proof-of-concept study tested the effects of obeticholic acid in patients with both Type 2 diabetes mellitus and nonalcoholic fatty liver disease with increased insulin sensitivity and reduced markers of liver inflammation and fibrosis (34).
Problems encountered with obeticholic acid are association with increased concentrations of total serum cholesterol and LDL cholesterol and decreased concentrations of HDL cholesterol. These changes developed within 12 wk of the start of treatment, decreased in magnitude during treatment, and were not sustained upon treatment discontinuation (36).
Conclusion
BAs may find a role in clinical therapeutics for a variety of diseases. The clearest therapeutic effects are in restoring normal bowel functions and among liver diseases for cholestatic or nonalcoholic fatty liver disease. There may be a potential therapeutic role in treatment of metabolic diseases including hyperlipidemia and Type 2 diabetes mellitus. Many of these effects involve a role of FXR and TGR5 modulation in these diverse diseases. Future research will consolidate the roles of BAs and their agonists and antagonists or sequestrants for these diverse indications.
GRANTS
This work was funded by NIH RO1-DK92179 (M. Camilleri, Principal Investigator) and NIH R01-DK41876 (G. J. Gores, Principal Investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.C. and G.J.G. drafted manuscript; M.C. and G.J.G. edited and revised manuscript; M.C. and G.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144: 145–154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci 52: 1270–1278, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl− secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol 305: C447–C456, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 282: G443–G449, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro 27: 964–977, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res 11: 120–123, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 29: 625–651, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517: 205–208, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M. Invited Review: Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol 8: 49–61, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, Lueke A, Gray A, Donato LJ. Effect of colesevelam on fecal bile acids and bowel functions in diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 41: 438–448, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M, Klee EW, Shin A, Carlson P, Li Y, Grover M, Zinsmeister AR. Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Physiol Gastrointest Liver Physiol 306: G13–G26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Physiol Gastrointest Liver Physiol 307: G508–G516, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med 94: 661–674, 1979. [PubMed] [Google Scholar]
- 14.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of A3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol 106: 1803–1812, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21: 159–165, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Bañares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, Viver JM. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 46: 2231–2238, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab 12: 384–392, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol 15: 567–582, 1986. [PubMed] [Google Scholar]
- 20.Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol 102: 1799–1807, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148: 751–761.e8, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159: 2647–2658, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res 55: 1553–1595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou R, Goldberg AC. Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety. Endocrinol Metab Clin North Am 38: 79–97, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103: 3920–3925, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsen TH, Vesterhus M, Boberg KM. Review article: Controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther 39: 282–301, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Keating N, Mroz MS, Scharl MM, Marsh C, Ferguson G, Hofmann AF, Keely SJ. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. J Cell Mol Med 13: 2293–2303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly OB, Mroz MS, Ward JB, Colliva C, Scharl M, Pellicciari R, Gilmer JF, Fallon PG, Hofmann AF, Roda A, Murray FE, Keely SJ. Ursodeoxycholic acid attenuates colonic epithelial secretory function. J Physiol 591: 2307–2318, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuipers F, Groen AK. FXR: the key to benefits in bariatric surgery? Nat Med 20: 337–338, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66: 948–983, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindor KD. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. N Engl J Med 357: 1524–1529, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 50: 808–814, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazaki-Anzai S, Masuda M, Levi M, Keenan AL, Miyazaki M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One 9: e108270, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145: 574–582, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 22: 390–400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E NASH. Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385: 956–965, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 8: 159–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol 127: 887–894, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol 15: 1677–1689, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol 36, Suppl 1: S3–S12, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Rao AS, Wong B, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 139: 1549–1558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 30: 332–338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Sonne DP, Hansen M, Knop FK. Bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. Eur J Endocrinol 171: R47–R65, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 21: E660–E668, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Stolk MF, Van Erpecum KJ, Peeters TL, Samsom M, Smout AJ, Akkermans LM, Vanberge-Henegouwen GP. Interdigestive gallbladder emptying, antroduodenal motility, and motilin release patterns are altered in cholesterol gallstone patients. Dig Dis Sci 46: 1328–1334, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Stotzer PO, Abrahamsson H, Bajor A, Sadik R. Effect of cholestyramine on gastrointestinal transit in patients with idiopathic bile acid diarrhea: a prospective, open-label study. Neuroenterology 2: 235657, 2013. [Google Scholar]
- 50.Taur Y, Pamer EG. Harnessing microbiota to kill a pathogen: fixing the microbiota to treat Clostridium difficile infections. Nat Med 20: 246–247, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triantos CK, Koukias NM, Nikolopoulou VN, Burroughs AK. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther 34: 901–910, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther 41: 54–64, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, Le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol 7: 1189–1194, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Wang DQ, Carey MC. Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol 20: 9952–9975, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis 5: e996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong BS, Camilleri M, Carlson PJ, Guicciardi ME, Burton D, McKinzie S, Rao AS, Zinsmeister AR, Gores GJ. A klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology 140: 1934–1942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 106: 2154–2164, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, Williamson C, Walters JR. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol 304: G940–G948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]