Abstract
The interleukin (IL)-23/IL-17A/IL-22 cytokine axis plays a critical role in the pathogenesis of psoriasis, wherein IL-22 effects on epidermal alternations by inhibiting differentiation and inducing keratinocyte proliferation. In this study, we investigated effects of curcumin on IL-22-induced proliferation in a human keratinocyte cell line (HaCaT) in vitro experiment. The HaCaT cells growth was measured by a Cell Counting Kit-8. The cyclin D1 and cyclin E was detected by real-time RT-PCR and weatern blot. The STAT3 and phosphorylation of STAT3 was tested by weatern blot also. Our results show that curcumin exhibited a significant anti-proliferation effect on HaCaT cells, even in the presence of IL-22. Since STAT3 is crucial for IL-22 signal transduction, we examined the level of phosphorylation of STAT3 in all of the experimental groups. As expected, curcumin inhibited IL-22 induced phosphorylation of STAT3; furthermore, curcumin down regulated cyclin D1 and cyclin E. We can reach a conclusion that curcumin can suppress the proliferation of keratinocytes even with IL-22 treatment. Therefore, we have confidence in future curcumin research about psoriasis treatment.
Keywords: Curcumin, keratinocyte, cell growth, STAT3, cyclin D1, cyclin E
Introduction
The interleukin (IL)-23/IL-17A/IL-22 cytokine axis plays a critical role in the pathogenesis of psoriasis. Skin lesions and the plasma of psoriasis patients show increased IL-22 production [1,2]. IL-22 is one of important cytokines produced by epidermal gd T cells, whose elevated plasma levels relate to disease severity [3]. IL-22 plays a pivotal role in the epidermal alterations of psoriasis, including acanthosis, hypogranularity and hyperkeratosis [3,4], which ascribe to the abnormal differentiation, proliferation, and migration of keratinocytes after the activation of the IL-22 signal transduction pathway [3,5,6]. Obviously, IL-22 is a potential target for psoriasis treatment.
Curcumin is a compound obtained from turmeric and investigated in-depth [7]. We found that curcumin was able to inhibit the hyper-proliferation of epidermal cells in an imiquimod-induced psoriasis mouse model [8]. The mechanism of curcumin inhibitory keratinocyte hyper-proliferation still remains unknown. Therefore, we designed this preliminary study to explore that role of curcumin using a human keratinocyte cell line (HaCaT).
Materials and methods
Chemicals
The reagents used in this study include recombinant human IL-22 (PeproTech, London, UK), curcumin and anti-b-actin antibody (Sigma, St. Louis, MO, USA), Cell Counting Kit-8 (CCK-8 Dojindo, Kumanoto, Japan), TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and PrimeScript RT Reagent Kit and SYBR® Premix Ex TaqTM II (Takara, Dalian, China). Anti-STAT3 antibody and anti-STAT3 phospho Y705 antibody were purchased from Abcam (Cambridge, UK). Antibodies against human cyclin D1 and cyclin E were purchased from Cell Signaling Technology (Boston, MA, USA) and Novus Biological (Littleton, CO, USA), respectively. Curcumin was dissolved in 40% propylene glycol PBS solution, stored at 4°C and further diluted in medium prior to each experiment.
Cell culture
The HaCaT cell line was maintained in our laboratory [9]. The HaCaT cells were cultured and passaged in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and penicillin (100 U/mL)/streptomycin (100 μg/mL). The HaCaT cells were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Cell growth curve assays
HaCaT cells were planted in 96-well plates (5 × 103 per well). Before the test, the cell culture supernatants were removed. The cells were washed twice with PBS, and tested by Cell Counting Kit-8 which measure the living cells. All of the procedure was followed the introduction of Kit. The absorbance values were measured spectrophotometrically by a multi-well scanning reader.
Western blot
To determine the levels of STAT3, p-STAT3, cyclin D1 and cyclin E expression in HaCaT cells, the total proteins were extracted as described previously [10]. A total of 20-40 μg protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), separated by electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with primary antibody followed by incubation with a species-appropriate secondary antibody coupled to horseradish peroxidase. The bands were visualized using an ECL detection kit and analyzed using Image Pro-Plus (6.0) [parameter: integrated optical density (IOD)]. The ratios of p-STAT3 to total STAT3 were calculated and compared in all groups.
Real-time RT-PCR
To detect the mRNA levels of cyclin D1 and cyclin E in HaCaT cells, real-time RT-PCR was performed as described previously [8]. The primers used in this experiment are showed in Table 1.
Table 1.
Primer sequences of human genes examined by quantitative real-time RT-PCR
| Target gene | Primer sequence |
|---|---|
| cyclinD1 (S) | 5’-GCTGGAGCCCGTGAAAAAGA-3’ |
| cyclinD1 (AS) | 5’-CTCCGCCTCTGGCATTTTG-3’ |
| cyclin E (S) | 5’-ATCAGCACTTTCTTGAGCAACA-3’ |
| cyclin E (AS) | 5’-TTGTGCCAAGTAAAAGGTCTCC-3’ |
| GAPDH (S) | 5’-CATGAGAAGTATGACAACAGCCT-3’ |
| GAPDH (AS) | 5’-AGTCCTTCCACGATACCAAAGT-3’ |
AS, antisense; S, sense.
Statistics
All experiments were performed in triplicate with a minimum of 3 replicates. The means and standard deviations were also calculated. The differences among treatments was determined by ANOVA analysis; and differences between treatments was analyzed by SNK test. A P-value of 0.05 was considered statistically significant. All of the data was processed with software of SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Curcumin inhibits the proliferation of IL-22-treated HaCaT cells
The growth curves of the HaCaT cells were recorded as described previously [11]. The HaCaT cells were exposed to a combination treatment of 7.37 μg/mL curcumin and 20 or 100 ng/mL IL-22. After 28 h, the absorbance values of the living cells in the IL-22 treatment (100 ng/mL) group increased remarkablly compared with the initial absorbance, but there was no significant difference in the IL-22 (100 ng/mL) and curcumin treatment groups, as presented in Figure 1A and 1B. However, there was no significant difference between the IL-22-treated group and the control group (P > 0.05). This result may due to the high rate of HaCaT cells proliferation, which covered the effect of IL-22. As expected, curcumin showed a strong inhibitory effect on HaCaT cells proliferation with or without IL-22 treatment. Moreover, the proliferation curves of HaCaT cells showed cyclical feature roughly. All results of statistic analysis are presented in Tables 2 and 3 of supplementary data file.
Figure 1.
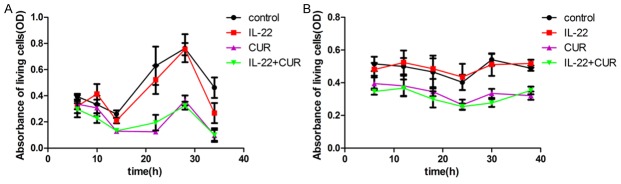
Growth curve of HaCaT cells. The proliferation of HaCaT cells was examined by a CCK-8 kit described in the Materials and Methods. The measurements were performed at the indicated time points. HaCaT cells were incubated with 7.37 μg/mL curcumin for 2 h and then incubated with IL-22 until the test was performed. A. Growth curve of HaCaT cells with IL-22 treatment (20 ng/mL). This test was performed at 6, 10, 14, 22, 28 and 34 h. B. Growth curve of HaCaT cells with IL-22 treatment (100 ng/mL). This test was performed at 6, 12, 18, 24, 30 and 38 h. All of the results are showed by mean±SD with sample size of 5. CUR, curcumin.
Table 2.
Results of statistic analysis from data of Figure 1A
| Group | 6 h | 10 h | 14 h | 22 h | 28 h | 34 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| All groups | 3.56 | 0.0382 | 7.37 | 0.0026 | 50.34 | < 0.01 | 20.42 | < 0.01 | 85.76 | < 0.01 | 36.30 | < 0.01 |
| CUR vs Control | - | > 0.05 | - | > 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 |
| IL-22 vs Control | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 |
| IL-22 vs IL-22+CUR | - | > 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 |
F, F value; P, P value; CUR, curcumin.
Table 3.
Results of statistic analysis from data of Figure 1B
| Group | 6 h | 12 h | 18 h | 24 h | 20 h | 38 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| All groups | 33.91 | < 0.01 | 13.18 | < 0.01 | 14.42 | < 0.01 | 18.87 | < 0.01 | 68.66 | < 0.01 | 136.96 | < 0.01 |
| CUR vs Control | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 |
| IL-22 vs Control | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 | - | > 0.05 |
| IL-22 vs IL-22+CUR | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 | - | < 0.05 |
F, F value; P, P value; CUR, curcumin.
Effect of curcumin on activation of STAT3, cyclin D1 and cyclin E
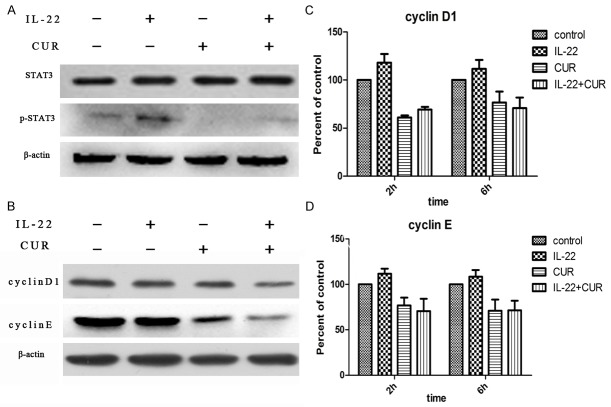
The levels of STAT3 and phosphorylated STAT3 (p-STAT3) protein were examined by western blot. As results showed that IL-22 was able to up-regulate the level of p-STAT3 protein remarkablly; and that effect of IL-22 was almost entirely inhibited by curcumin (Figure 2A). Furthermore, the p-STAT3 level in normal HaCaT cells was down-regulated by curcumin as well. There was no difference of STAT3 levels observed in all groups. The levels of cyclin D1 and cyclin E in HaCaT cells were examined by real-time RT-PCR and western blot. The results showed that both cyclin D1 and cyclin E levels were suppressed by curcumin even with or without IL-22 treatment (Figure 2B). But we failed to find any difference of cyclin D1 and cyclin E mRNA levels in all groups (Figure 2C and 2D).
Figure 2.
Effects of curcumin on STAT3 and cyclins. A. Curcumin inhibits STAT3 activation. HaCaT cells were incubated with curcumin (7.37 μg/mL) for 2 h and then treated with IL-22 (100 ng/mL) for 30 min before performing western blot assay. B. The expression of cyclin D1 and cyclin E. HaCaT cells were incubated with curcumin (7.37 μg/mL) for 2 h and then treated with IL-22 (100 ng/mL) for 24 h before performing western blot assay. C. The mRNA level of cyclin D1. HaCaT cells were incubated with curcumin (7.37 μg/mL) for 2 h and then treated with IL-22 (100 ng/mL) for 2 h or 6 h. D. The mRNA level of cyclin E. The experimental details are the same as those for cyclin D1. The results shown are representative of at least 3 independent experiments. CUR, curcumin.
Discussion
Psoriasis is a chronic skin disease characterized by keratinocyte hyper-proliferation and a massive infiltration of leukocytes [2]. The over-expression of IL-22 is a key factor in the pathogenesis of psoriasis vulgaris, which targets keratinocytes. Therefore, the antagonism of IL-22 is an attractive idea in psoriasis research. Interestingly, we found that curcumin possessed this antagonism role in previous study [8] and verified again in this study. As discussed above, we previously found that curcumin inhibits the hyper-proliferation of keratinocytes in an imiquimod-induced psoriasis mouse model. The topical application of imiquimod on mouse skin leads to activation of the IL-23/IL-17A/IL-22 axis [12]. In this study, we found that curcumin displayed a strong inhibitory effect on HaCaT cells proliferation with or without IL-22 treatment. As we know IL-22 signal conduction in keratinocytes involves JAK-STAT3 pathway. And inhibitory of STAT3 activation was able to attenuate a psoriasis-like phenotype in a mouse model [13]. Our study indicated that p-STAT3 was up-regulated by IL-22-treatment, which was suppressed significantly by curcumin. Furthermore, we found that curcumin suppressed the expression of cyclin D1 in IL-22 treated HaCaT cells. That indicated the target of curcumin inhibited cell proliferation by impact on cell cycle. However, as a signal marker of cell growth, cyclin D1 was not abnormally expressed in IL-22-treated HaCaT cells. This result is consistent with the indication of cell growth curves. Since cyclin E is expressed downstream of cyclin D in the IL-22-activated Erk/MAPK pathway, it is reasonable that the expression of cyclin E was significantly reduced either in curcumin-treated HaCaT cells. That suggested curcumin may inhibit the proliferation of HaCaT cells by stoping cells in S phase. Moreover, our results showed that both the cyclin D1 and cyclin E protein levels, but not the mRNA levels, were suppressed by curcumin. This finding suggested that curcumin might impact cyclin D1 and cyclin E protein expression on post-transcriptional modifications.
HaCaT cells are an immortalized cell line that proliferates quickly with stimulation of serum growth factors. The role of IL-22 in promoting HaCaT cells proliferation may be covered by their high proliferation. In spite of it is seemingly more appropriate to use primary keratinocyte cells with a slow growth speed. However, primary keratinocyte cells must be grown in serum-free keratinocyte medium, which induce poor comparable results due to difference from physical environment. In conclusion, curcumin can suppress the proliferation of keratinocytes with IL-22 or without IL-22 treatment. Anyway, additional in-depth studies are necessary in future.
Disclosure of conflict of interest
None.
References
- 1.Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011;187:5026–5031. doi: 10.4049/jimmunol.1101817. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 5.Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, Cavani A, Girolomoni G, Albanesi C. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–927. doi: 10.1096/fj.10-172288. [DOI] [PubMed] [Google Scholar]
- 6.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 7.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 8.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8:e67078. doi: 10.1371/journal.pone.0067078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Han J, Zhu Q, Li Z, Hu J. Camptothecin fails to induce apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Pharmacology. 2012;89:58–63. doi: 10.1159/000335370. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Han J, Zhao Y, Zhu Q, Hu J. Curcumin induces apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Int Immunopharmacol. 2012;13:170–174. doi: 10.1016/j.intimp.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2006;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 12.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S. Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest Dermatol. 2011;131:108–117. doi: 10.1038/jid.2010.255. [DOI] [PubMed] [Google Scholar]