Abstract
MicroRNAs (miRNAs) are small, non-coding RNAs that modulate gene expression by negatively regulating the stability or translational efficiency of their target mRNAs. The aim of this study was to investigate the expression of microRNA-145 (miR-145) in human papillary thyroid cancer and its potential function. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to determine the expression level of miR-145 in ten papillary thyroid cancer and adjacent normal specimens. The function of miR-145 overexpression on the proliferation of human TPC1 thyroid cancer cells was conducted by MTT assays and by colony-formation assays. Western blot was used to validate the impact of miR-145 on the protein expression of the target gene. Luciferase reporter assays were employed to validate a putative target of miR-145. MiR-145 expression was relatively decreased in papillary thyroid cancer specimens compared with adjacent normal tissues (P<0.05). MTT assays and colony-formation assays showed that overexpression of miR-145 suppressed TPC1 cell growth. Luciferase assays using a reporter carrying a putative miR-145 target site in the 3’ untranslated region of DUSP6 revealed that miR-145 directly targets DUSP6. Overexpression of miR-145 led to downregulation of DUSP6 at protein level as assessed by Western blot. Targeted knockdown of DUSP6 by siRNA significantly inhibited the proliferation of TPC1 cells. The overexpression of miR-145 inhibited TPC1 cellular growth by targeting DUSP6; this finding implies a better understanding of initiation and progression of papillary thyroid cancer.
Keywords: MiR-145, proliferation, DUSP6, papillary thyroid cancer
Introduction
Thyroid cancer was the most common endocrine cancer in the USA and in 2010. There were 44670 new diagnoses of this disease [1]. Thyroid cancer tumors are primarily classified into four types and among these four types, papillary thyroid cancer (PTC) is the most common malignant thyroid cancer in countries with sufficient iodine diets, comprising up to 80% of all thyroid malignancies [2].
MiRNAs are small endogenous non-coding RNAs that negatively regulate gene expression by degrading mRNAs of target genes or blocking their translations [3]. In PTCs, there are an increasing amount of evidence suggesting that miRNAs are implicated in the carcinogenesis of PTCs. For instance, miR-34a targets GAS1 to promote cell proliferation and to inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad Pathway [4], miR-199a-3p displays tumor suppressor function in papillary thyroid carcinoma [5], and miRNA-101 suppresses migration and invasion by targeting Rac1 in thyroid cancer cells [6].
It was reported that miR-145 suppresses thyroid cancer growth and metastasis by targeting AKT3 [7]. However, whether miR-145 regulates other target genes through a different cell signalling pathway in papillary thyroid cancer remains unknown. In order to explore whether miR-145 has another potential target gene, we searched several online databases, including targetscan, miRanda, miRBase, and miRGen, confirmed our premise that DUSP6 mRNA contains miR-145 binding sites.
The protein encoded by DUSP6 gene is a member of the dual specificity protein phosphatase subfamily. These phosphatases inactivate their target kinases by dephosphorylating both the phosphoserine/threonine and phosphotyrosine residues. In addition, these phosphatases negatively regulate members of the mitogen-activated protein (MAP) kinase superfamily (MAPK/ERK, SAPK/JNK, p38), which are associated with cellular proliferation and differentiation (http://www.ncbi.nlm.nih.gov/gene/1848). In different thyroid cancer cell lines, DUSP6 is highly expressed [8] and in clinical specimens, DUSP6/MKP3 cytoplasmic phosphatase is overexpressed in papillary thyroid carcinoma [8]. MAP kinase phosphatase enzymes (MKPs) belong to the family of dual-specificity phosphatases (DUSPs), inactivate different MAPK proteins that include ERK1/2, which take part in tumorigenesis, especially in papillary thyroid cancer via the MAPK pathway; this is a very important signal transduction pathway for PTC [9,10]. In this study, we found overexpression of miR-145 inhibited cells’ growth, most likely targeting DUSP6. These results come to a conclusion that miR-145 may function as a tumor suppressor, which implies a better understanding of the initiation and progression of papillary thyroid cancer.
Materials and methods
Specimens
From the Department of General Surgery of the Shanghai Tenth People’s Hospital (Shanghai, China), we collected 10 thyroid papillary cancer specimens that were paired with adjacent normal thyroid papillary tissues collected. The samples were immediately snap-frozen in liquid nitrogen. All samples were pathologically confirmed as thyroid papillary cancer by trained pathologists. No patients received chemotherapy or radiotherapy prior to surgery. The collection of the patient specimens were approved by Institutional Ethics Committees of Tongji University (approval number: SHSY-IEC-pap3.0/13-3).
Immunohistochemistry
Avidin-biotinylated peroxidase complex (ABC) immunohistochemistry was performed according to the method described [11] as follows: 4-mm thick sections were cut from paraffin blocks of PTC. After deparaffinization and rehydration, we performed heat-induced antigen retrieval by autoclave pretreatment (120°C for 10 min) in citrate buffer solution (pH 6.0) on the sections. Endogenous peroxidase activity was blocked by immersing the slides in absolute methanol solution containing 3% hydrogen peroxide (H2O2) for 10 min. Endogenous biotin activity was blocked using an avidin/biotin blocking kit purchased from NICHIREI, (Tokyo, Japan). Sections were incubated in avidin solution for 15 min followed a by brief rinse in PBS, after which sections were incubated in biotin solution for 15 min (all performed at room temperature). Next, sections were treated with 1% bovine serum albumin for 30 min to block nonspecific reactions, after which they were incubated with DUSP6 antibody (1:1000 dilution). Following incubation, specimens were visualized with an ABC detection kit (Vector laboratory, Burlingame, CA) and a diaminobenzidine (DAB) substrate system, according to the instructions provided by the manufacturer. All reactions were performed using appropriate positive and negative controls, and no significant staining was observed in the negative control sections. Image pro-plus 6.0 software was used to analyze integrated optical density of positive value (IOD) and the total area of the organization (AREA) of each photo (Mean Density = IOD/AREA).
Cell lines and transfection
The human papillary cancer cell line TPC1 and normal thyroid cell line Nthy-ori 3-1 were obtained from Chinese Science Institute (Shanghai, China). The TPC1 cancer cells and Nthy-ori 3-1 normal cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), penicillin (100 U/mL) and streptomycin (100 µg/mL) (Enpromise, China). Cells were incubated at 37°C in a humidified chamber supplemented with 5% CO2. MiR-145 mimics, control mimics (NC), DUSP6 siRNA, and scramble siRNA were chemosynthesized by Shanghai Genepharma Co, Ltd. (Shanghai, China). The TPC1 cells were cultured to 30% to 40% confluence in 6-well plates and were transfected with miR-145 mimics or DUSP6 siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer’s instructions. miR- and siRNA-negative control (NC) were used as negative controls.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
For detection of miR-145 expression, primer design and qRT-PCR were carried out. miRNA was isolated from tissues and cells using the miRcute miRNA Isolation kit according to the manufacturer’s instructions (Tiangen, China). The primers for miR-145 were stem-loop RT primer: 5’-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAA-TTGCACTGGATACGACAGGGATT-3’ (stem-loop primer). miR-145 forward: 5’-GGTCCAGTTTTCCCAGG-3’; miR-145 reverse: 5’-CAGTGCGTGTCGTGGAGT-3’. The primers for U6 small nuclear RNA were RT primer 5’-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGG-3’; forward 5’-TGCGGGTGCTCGCTTCGGCAGC-3’ and reverse 5’-CCAGTGCAGGGTCCGAGGT-3’.
cDNA was generated by reverse transcription using the Prime Script™ RT-PCR kit in accordance with the manufacturer’s instructions (Takara, Tokyo, Japan). Real-time PCR was performed on a 7900HT Fast RT-PCR instrument (Applied Bio systems, Singapore). The amplification procedure was as follows: 5 min at 95°C, followed by 40 cycles at 95°C for 30 sec and 65°C for 45 sec. Each sample was tested in triplicate, and the fold-change of mRNA expression was calculated using the 2-ΔΔCt method [12].
Cell proliferation assay
Cell proliferation was determined using an MTT assay kit (Sigma, Santa Clara, CA, USA) in accordance with the manufacturer’s instructions. Briefly, cells (1×103 cells/well) that transfected with miR-145, DUSP6 siRNA mimics and the corresponding NC mimics were seeded into 96-well culture plates (BD Biosciences, Franklin Lakes, NJ, USA) and incubated overnight at 37°C in 5% CO2. Cell proliferation was assessed at 24, 48, 72 and 96 h following addition of 0.5 mg/mL MTT (Sigma) solution. After a 4-h incubation, the medium was replaced with 150 µL dimethylsulfoxide (DMSO; Sigma) and vortexed for 10 min. The optical density (OD) of each well was measured using a microplate reader (Biotek, Vermont, America) at 490 nm. Each experiment was performed in triplicate.
Colony formation assay
MiR-145 mimic, control mimic, DUSP6 siRNA and scramble siRNA are the same with MTT assay. A full 24 h after transfection, TPC1 cells were digested with trypsin and suspended into a single cell status. There were 500 cells from each group cultured in the 6-well plate for 14 days. When the colonies were visible by eye, the culture was terminated by removing the medium and washing cells twice with phosphate-buffered saline (PBS). The colonies were fixed with 95% ethanol for 10 min, dried and stained with 0.1% Crystal Violet solution for 10 min, and the plate was washed three times with water. Colonies less than 2 mm in diameter and faintly stained were ignored. Each treatment was performed in triplicate.
Dual-luciferase reporter assay
HEK293T cells were seeded in 12-well plates (BD, USA) and cultured until the cells reached 80-90% confluence. The 3’-UTR segments of the DUSP6 mRNA sequence containing the predicted miR-145 binding sites were amplified by PCR in a total volume of 50 µL using the PrimerStar kit (Takara, Tokyo, Japan) in accordance with the manufacturer’s instructions. The corresponding mutant constructs were created by mutating the seed regions of the miR-145-binding sites (5’-AACUGGAA-3’ to 5’-UUGACCUU-3’). The mutant constructs were generated by mutation. The primers used in the reaction were (Forward: 5’-AGTAATTCTAGGCGATCGCTCGAGAAGAAA-3’; Reverse: 5’-GATATTTTATTGCGGCCAGCGGCCGCTAAA-3’). Fragments were subcloned into the XhoI site in the 3’-UTR of Renilla luciferase of the psiCHECK-2 reporter vector. 293T cells were transiently cotransfected with 0.2 µg psiCHECK-2/DUSP6 3’-UTR or psiCHECK-2/DUSP6 3’-UTR mutant reporter plasmids and together with 100 nmol/L miR-145 or miR-NC using Lipofectamine™ 2000 (Invitrogen), according to the manufacturer’s instructions. After 48 h, firefly and Renilla luciferase activities were measured by using a Dual Luciferase Assay (Promega, Madison, WI, USA). Firefly luciferase values were normalized to Renilla, and the ratio of firefly/renilla was presented.
Western blot analysis
Cells were lysed in lysis buffer (10 mmol/L Tris-HCl, pH 7.4, 1% NP-40, 0.1% deoxycholic acid, 0.1% SDS, 150 mmol/l NaCl, 1 mM EDTA and 1% protease inhibitor cocktail) (Sigma). The protein concentrations were quantified using a BCA protein assay kit (Pierce). Protein (30 µg) was separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Beyotime Institute of Biotechnology, Jiangsu, China). The membrane underwent immunoblotting overnight at 4°C with primary antibodies against DUSP6 (1:1,000; Abcam, USA) and β-tubulin (1:2,000; Cwbio, Jiangsu, China), as loading controls. Horseradish peroxidase-conjugated secondary antibodies were incubated with the membrane for 30 min at 37°C after 3 washes with TBST. Immunoreactive protein bands were detected with an Odyssey Scanning system.
Statistical analysis
GraphPad Prism version 6.0 (GraphPad, San Diego, CA, USA) was used for all statistical analyses. Data were presented as the means ± standard deviation (SD) from at least 3 separate experiments. The t-test (two-tailed) was used to draw a comparison between groups, and the significance level was set at P<0.05.
Results
Expression of miR-145 was lower in papillary thyroid cancer specimens than adjacent normal tissues
The expression levels of miR-145 were measured by quantitative polymerase chain reaction (qPCR) in 10 thyroid papillary cancer tissues and para-cancer tissues. The results showed that the expression of miR-145 was lower in thyroid papillary cancer tissues compared with para-cancer tissues (P<0.05) (Figure 1).
Figure 1.

Expression of miR-145 in papillary thyroid cancer: MiR-145 levels are significantly decreased in thyroid cancer specimens based on qPCR (P<0.05).
Expression of DUSP6 was higher in papillary thyroid cancer tissues and TPC1 cells
We examined the DUSP6 expression in TPC1 cancer cells and Nthy-ori 3-1 normal thyroid cells at protein level. As shown in Figure 2A, expression of DUSP6 is significantly higher in TPC1 cells compared with the levels in the Nthy-ori 3-1 normal thyroid cells. The immunohistochemical staining of PTC samples confirmed that DUSP6 expression was higher in papillary cancer tissues than in para-cancer tissues (Figure 2B and 2C).
Figure 2.

Expression of DUSP6 in TPC1 cells and in clinical samples: A. The expression of DUSP6 was increased in TPC1 thyroid papillary cancer cells by Western blot, compared with Nthy-ori 3-1 normal thyroid cells. B and C. In clinical samples, DUSP6 was also highly expressed.
miR-145 suppresses proliferation of TPC1 cells
We transfected TPC1 cells with miR-145 mimics or negative control (NC) mimics to determine overexpression of miR-145 on TPC1 cell proliferation, performed by MTT assays and Colony formation assays. Overexpression of miR-145, transfected with 100 nM miR-145 mimics, suppressed significantly proliferation of TPC1 cells, compared with the NC group from 48 h until 96 h, under time-dependent conditions (Figure 3). This was detected by MTT assays that optical density (OD) values at 490 nm. Colony formation assays showed much less colony formation in the group transfected with 100 nM miR-145 compared with the NC group (42±2 miR-145 group vs. 93±3 NC group, P<0.05) (Figure 4A and 4B). These results indicated that overexpression of miR-145 suppressed TPC1 cellular proliferation.
Figure 3.
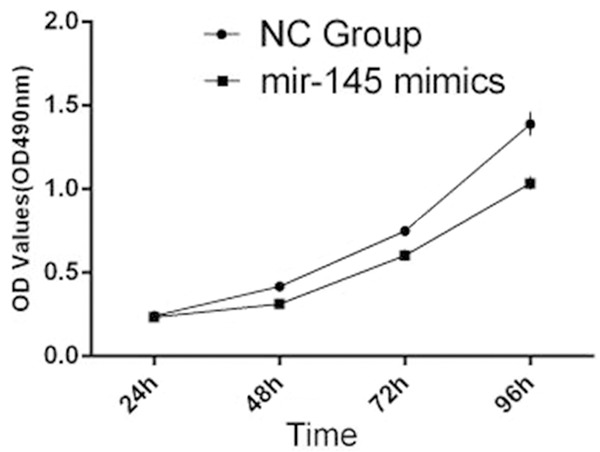
MTT cell proliferation assays: The proliferation of TPC1 cells transfected with 100 nm miR-145 mimics was inhibited in a time dependent manner compared with the NC group conducted by MTT cell proliferation assays.
Figure 4.
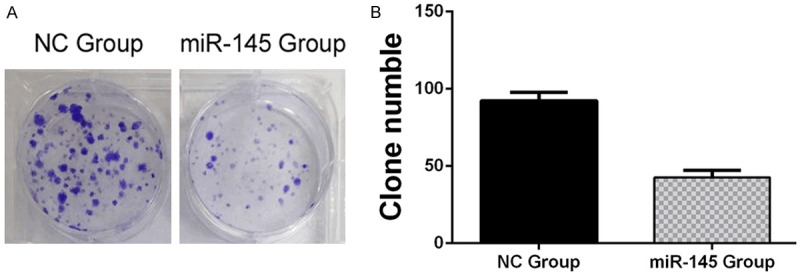
Colony formation assays: Overexpression of miR-145 inhibited colony formation ability of TPC1 cells. A. Colony formation assays showed Crystal Violet staining of the miR-145-transfected group and NC-transfected group. B. Graph indicates colony numbers of each experimental transfected group (P<0.05).
MiR-145 targets DUSP6 and regulates its expression in TPC1 cells by binding the 3’UTR of DUSP6
To investigate whether DUSP6 is another potential targets of miR-145, we searched several online databases, including targetscan, miRanda, and miRBase. All databases showed that miR-145 had a binding site in the 3’-UTR of DUSP6 mRNA. To verify miR-145 binding to this predicted site, we performed a luciferase reporter assay in the 293T cell line. We cloned the 3’-UTR of DUSP6 containing the putative miR-145 binding site into a luciferase reporter construct, in addition to a mutated DUSP6 3’-UTR (Figure 5A). The results (Figure 5B) showed that the luciferase activity significantly decreased after co-transfection with psiCHECK-2/DUSP6 3’-UTR. In addition, miR-145 mimics control cells, demonstrating that miR-145 specifically binds to the 3’-UTR of DUSP6 mRNA. Furthermore, as shown in Figure 5C, Western blot analysis indicated that DUSP6 protein levels were lower in the miR-145-overexpressing group compared with the NC group.
Figure 5.
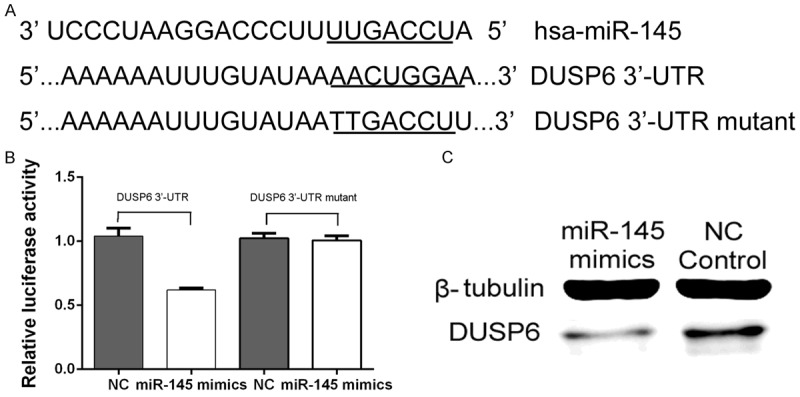
DUSP6 is a target of miR-145: miR-145 downregulates DUSP6 expression by binding the 3’-UTR of DUSP6. A. The binding site for miR-145 in the 3’-UTR of DUSP6 mRNA. B. The relative luciferase activity (Renilla/firefly) was measured in HEK293T cells after co-transfection of the DUSP6 3’-UTR or DUSP6 3’-UTR mutant luciferase construct with either miR-145 mimics or miR-NC. C. Western blot analysis was used to detect DUSP6 protein expression levels.
MiR-145 inhibits the proliferation of papillary cancer cells via regulation of DUSP6
DUSP6 is a direct target of miR-145, confirmed by Dual-luciferase reporter assay. Therefore, we supposed that miR-145 suppresses the proliferation of TPC1 cancer cells might be achieved by targeting DUSP6. To investigate this, we knocked down DUSP6 expression by transfecting 100 nmol/L DUSP6 siRNA (siDUSP6) to TPC1 cells and checked its impact on the growth of TPC1 cells. The protein level of DUSP6 was significantly downregulated in siDUSP6 treatment group (Figure 6A) and cellular proliferation was significant inhibited, compared with control siRNA, conducted by MTT assays (Figure 6B) and Colony formation assays (38±3 siDUSP6 group vs. 85±4 NC group, *P<0.05) (Figure 6C and 6D). These results demonstrated that overexpression of miR-145, at least partially by downregulation of DUSP6 expression, inhibited proliferation of TPC1 thyroid cancer cells.
Figure 6.
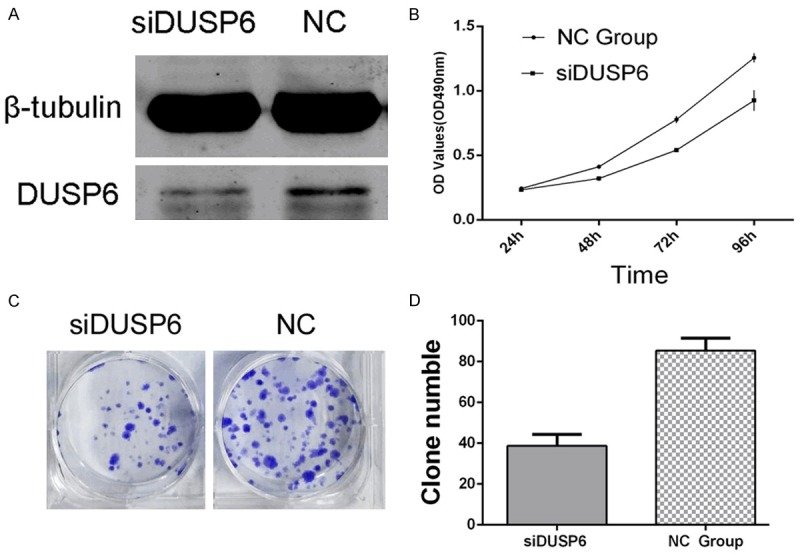
Knockdown of DUSP6 suppresses the proliferation of TPC1 cells: A. DUSP6 siRNA remarkably silenced the expression of DUSP6. B-D. MTT assays and Colony formation assays showed that siDUSP6 significantly suppressed the cellular proliferation.
Discussion
The functions of miRNAs have been studying in a wide range of physiological processes, especially in various cancer processes, including proliferation, apoptosis, and metastasis [13,14]. Papillary thyroid cancer is the most common type of thyroid cancer and is influenced by various miRNAs [15]. In our study, we found that miR-145 expression was significantly downregulated in papillary thyroid cancer specimens compared with normal tissues. When miR-145 was transfected into TPC1 cells, cellular proliferation was significantly decreased, indicating the miR-145 suppresses the growth of papillary thyroid cancer cells. In another study, transfection of miR-145 inhibits cell growth and invasion of pancreatic cancer and enhances gemcitabine sensitivity because of reduction of HER2, P-AKT, PAK1, and an increase in p53 by targeting MUC13 [16]. It has been reported that miR-145 is able to inhibit cell proliferation and growth activity significantly by reducing mRNA and protein expression levels of A disintegrin and metalloproteinase 17 (ADAM17) in liver cancer cells. This is primarily an upstream component for multiple EGFR pro-ligands that bind with ligands subsequently activating MEK/ERK and PI3K/Akt pathways to contribute to invasiveness and other malignant phenotypes [17]. All of these studies illustrate that miR-145 might has multiple function in various cancers.
For exploring whether miR-145 has another potential target gene, we searched several online databases, including targetscan, miRanda, miRBase, and miRGen and found that the DUSP6 mRNA contained miR-145 binding sites. The interaction between miR-145 and DUSP6 mRNA has not been previously reported. To confirm the targeting of DUSP6 by miR-145, the luciferase reporter assay was conducted and it showed luciferase activity was significantly repressed in cells transfected with the construct harboring the miR-145 target sequence compared with the mutated control vector. Overexpression of miR-145 decreased DUSP6 protein expression level in TPC1 cells. Furthermore, silencing DUSP6 expression by siRNA resulted in the inhibition of the cellular proliferation of TPC1 cells. As reported that MAP kinase phosphatase enzymes (MKPs), which belong to the family of dual-specificity phosphatases (DUSPs), inactivate different MAPK proteins including ERK1/2, via MAPK pathway, so that take part in the tumorigenesis, especially in papillary thyroid cancer [9,10]. Our study revealed that miR-145 inhibited cellular growth in papillary thyroid cancer by targeting DUSP6, which may act through a newly determined and a classical signal pathway-the MAPK pathway and may provide additional evidences to further prove miR-145 is a potent tumor suppressor gene in papillary thyroid cancer.
Taken together, the expression level of miR-145 is relatively reduced in thyroid cancer specimens. miR-145 directly silences DUSP6 in thyroid cancer cells and regulates DUSP6 expression, which mechanically accounts for the suppression of cellular proliferation in papillary thyroid cancer. All data obtained in this study indicate that miR-145 might serve as a tumor suppressor gene in the carcinogenesis and maintenance of thyroid cancer.
Acknowledgements
This research was made possible with financial support from the National Natural Sciences Foundation of China, for the project 81272240, and the Shanghai Science Committee Found-ation (to L.F.) (No. STCSM 10411964700). Furthermore, we give special thanks to all the teachers at the Central Laboratory of the Shanghai Tenth People’s hospital for their technical assistance.
Disclosure of conflict of interest
None.
Abbreviations
- miRNA
microRNA
- NC
Negative Control
- qPCR
quantitative polymerase chain reaction
- nm
nmol/l
- DUSP6
Dual specificity phosphatase 6
- FBS
Fetal bovine serum
- DMEM
Dulbecco’s modified Eagle’s medium
- RL
Renilla luciferase
- FL
Firefly luciferase
References
- 1.Liebner DA, Shah MH. Thyroid Cancer: Pathogenesis and Targeted Therapy. Ther Adv Endocrinol Metab. 2011;2:173–195. doi: 10.1177/2042018811419889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacifico F, Leonardi A. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 2010;321:29–35. doi: 10.1016/j.mce.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Nikiforova MN, Chiosea SI, Nikiforov YE. MicroRNA expression profiles in thyroid tumors. Endocr Pathol. 2009;20:85–91. doi: 10.1007/s12022-009-9069-z. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Qin H, Cui Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013;441:958–963. doi: 10.1016/j.bbrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Minna E, Romeo P, De Cecco L, Dugo M, Cassinelli G, Pilotti S, Degl’Innocenti D, Lanzi C, Casalini P, Pierotti MA, Greco A, Borrello MG. miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma. Oncotarget. 2014;5:2513–2528. doi: 10.18632/oncotarget.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Lu S, Jiang J, Jia X, Dong X, Bu P. Hsa-microRNA-101 suppresses migration and invasion by targeting Rac1 in thyroid cancer cells. Oncol Lett. 2014;8:1815–1821. doi: 10.3892/ol.2014.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boufraqech M, Zhang L, Jain M, Patel D, Ellis R, Xiong Y, He M, Nilubol N, Merino MJ, Kebebew E. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21:517–531. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Yang C, Bojdani E, Murugan AK, Xing M. Identification of RASAL1 as a major tumor suppressor gene in thyroid cancer. J Natl Cancer Inst. 2013;105:1617–1627. doi: 10.1093/jnci/djt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degl’Innocenti D, Romeo P, Tarantino E, Sensi M, Cassinelli G, Catalano V, Lanzi C, Perrone F, Pilotti S, Seregni E, Pierotti MA, Greco A, Borrello MG. DUSP6/MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr Relat Cancer. 2013;20:23–37. doi: 10.1530/ERC-12-0078. [DOI] [PubMed] [Google Scholar]
- 10.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashiguchi M, Ueno S, Sakoda M, Iino S, Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, Ishigami S, Natsugoe S. Clinical implication of ZEB-1 and E-cadherin expression in hepatocellular carcinoma (HCC) BMC Cancer. 2013;13:572. doi: 10.1186/1471-2407-13-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Q, Wei C, Li X, Li J, Chen L, Huang Y, Song H, Li D, Fang L. MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncol Rep. 2014;31:1096–102. doi: 10.3892/or.2014.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sits and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Kong X, Zhang J, Luo Q, Li X, Fang L. MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell Int. 2013;13:7. doi: 10.1186/1475-2867-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuziwara CS, Kimura ET. MicroRNA Deregulation in Anaplastic Thyroid Cancer Biology. Int J Endocrinol. 2014;2014:743450. doi: 10.1155/2014/743450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, Yacoubian AM, Behrman SW, Zafar N, Kumar D, Thompson PA, Jaggi M, Chauhan SC. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget. 2014;5:7599–7609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Wu C, Wang Y, Wen S, Wang J, Chen Z, He Q, Feng D. MicroRNA-145 inhibits cell proliferation by directly targeting ADAM17 in hepatocellular carcinoma. Oncol Rep. 2014;32:1923–1930. doi: 10.3892/or.2014.3424. [DOI] [PubMed] [Google Scholar]


