Abstract
The effect of preimplantation exposure to bisphenol-A (BPA) on blastocyst development and implantation is investigated. Mice were orally administered with BPA (200, 400, 600, and 800 mg/kg/day) from Day 0.5 to Day 3.5 of their pregnancy. Blastocyst development was examined on Day 4 of pregnancy. With 400 mg/kg/day BPA, implantation site number and implantation rate significantly reduced. With 600 and 800 mg/kg/day BPA, no implantation site was observed. BPA at 800 mg/kg/day significantly reduced blastocyst development rate and hatching rate. With 400 and 600 mg/kg/day BPA, Blastocyst development rate showed no significant difference whereas hatching rate was lower. With 400, 600, and 800 mg/kg/day BPA, some embryos were detected in the fallopian tube and hatched blastocysts showed greatly increased apoptosis level and endothelial nitric oxide synthase expression. In summary, high concentration BPA delayed the transfer of embryos to the uterus, damaged blastocyst development before implantation, and inhibited embryo implantation.
Keywords: Bisphenol A, blastocyst development, embryo implantation, apoptosis, endothelial nitric oxide synthase
Introduction
Bisphenol-A (BPA) is one of the most widely used compounds in the industry. It is mainly used in the production of high-polymer materials, such as epoxy resin, polysulfone resin, polyphenylene ether resin and unsaturated polyester resin. BPA can affect human health and its main target organ is the reproductive system [1-4]. It is demonstrated that BPA reduces the sperm motility of male animals and damages spermatogenesis, thus reducing the fertility of male offspring and leading to decreased libido and abnormal sexual behaviors of male animals [5-7]. BPA promotes follicle atresia [8] and oocyte apoptosis [9], but inhibits the expression of steroidogenic enzyme in granulosa cells [10] and interrupts the production and secretion of steroid hormones [11]. For the uterus, BPA mainly influences uterus development at the perinatal period and implantation stage. Xiao et al. found that BPA changed receptivity of endometrium to embryos at the implantation stage [12]. The implantation process requires the synchronized development of embryos and endometrium. When BPA influences the receptivity of endometrium to embryos at the implantation stage, it is unclear whether BPA also influences blastocyst development before implantation, leading to loss of the intruding ability of hatched blastocysts in the endometrium.
For the development of normal embryos, a certain concentration of nitric oxide (NO) is needed to regulate the mitosis of embryonic cells [13]. As a key enzyme in the regulation of NO generation, expression of nitric oxide synthase (NOS) in embryonic cells varies according to the demands of embryos on the NO amount at different development stages [13]. eNOS and iNOS expressions have been detected in embryos at early stages [14]. Meanwhile, the expression of embryonic cells in NOS is also subject to exogenous hormones. Gouge et al. reported that exogenous estrogen improved expression of endothelial NOS (eNOS) and inducible NOS (iNOS) [15] in blastocysts. Saxena et al. found that estrogen could induce expressions of iNOS in endometrial epithelium cells, and the inducible effects could be enhanced by progesterone [16]. However, it is unknown whether BPA simulates the effect of estrogen, changes the NOS expression in embryos, and influences embryo development. Xiao et al. found that subcutaneous injection of 100 mg/kg/day BPA into pregnant mice inhibited embryo implantation [12]. Beger et al. orally administered BPA on pregnant mice and found that pregnancies of the mice were terminated upon daily intake of 68.84 mg of BPA [17]. The major pathway of BPA in the human body is oral administration. Thus, in this study, the effects of gavaged BPA at increasing concentrations on blastocyst development and implantation were analyzed.
Materials and methods
Chemicals and animals
All reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA) unless otherwise stated. The Kunming mice were purchased from the Changchun Hi-Tech Laboratory Animal Research Center (Changchun, China), and housed in a photoperiod (light/dark 14 hours: 10 hours). Animal experiments were carried out following the protocol approved by the Jilin Medical College Institutional Animal Care and Use Committee.
Animal treatments with BPA
The 2-month-old female mice were mated naturally with healthy fertile young males. The presence of a vaginal plug in the vaginal opening was considered as day 0.5 of pregnancy in the next morning. Not all females in which a plug was observed would become to be pregnant. Mice with the presence of embryos in the reproductive tract were considered pregnant. Pregnant females were randomly divided into five groups (n=30 per group). From 0.5 to 3.5 days in the pregnancy, the pregnant females were daily gavaged with 0, 200, 400, 600, and 800 mg/kg/day of BPA ((CH3)2C(C6H4OH)2) in the sesame oil.
In vivo development of blastocysts and staining of implantation sites
Uteri of pregnant mice untreated (gavaged with sesame oil only) and treated with BPA were flushed with PBS to detect the presence of embryos and the stages of embryo development at gestation day 4. To evaluate the embryo quality, the collected hatched blastocysts were stained with Hoechst33342 to determine blastomere number. On day 4.5 of pregnancy, the mice were anesthetized by intraperitoneal injection of chloral hydrate, and then intravenously injected with Chicago sky blue to identify implantation sites. The number of implantation sites was recorded. If no implantation sites were observed, the uterus of the mouse was flushed to detect the presence of embryos.
Immunostaining of blastocysts
Embryos were fixed in 4% paraformaldehyde in PBS for 40 min at room temperature, and then permeabilized with 0.2% Triton X-100 in PBS for 10 min. Immunocytochemical staining was performed by incubating the fixed samples with primary antibodies for 2 h, followed by secondary antibodies conjugated with FITC for 60 min. Polyclonal antibodies against active-caspase-3 (Abcam, Cambridge, MA, USA) and eNOS (Santa Cruz Biotechnology, Dallas, TX, USA) were diluted 1:200. The DNA was stained with 1 μM Hoechst33342. The embryos were then washed and mounted under a coverslip with gentle compression in VectaShield antibleaching solution (Vector Labs, Burlingame, CA, USA). Fluorescence was detected on a laser-scanning confocal microscopy (FV-500, Olympus, Japan). All images stained with the same antibody were taken at the same laser power.
Statistical analysis
The data were analyzed with SPSS (Statistics Production for Service Solution, Version 13.0) using one-way ANOVA. A probability of P < 0.05 was considered to be statistically significant.
Results
Inhibitory effect of the high-concentration BPA on embryo implantation
To determine the effect of BPA on embryo implantation, on Day 4.5 of pregnancy, staining was carried out at the implantation sites of healthy pregnant mice and BPA-treated pregnant mice (Figure 1). The respective numbers of implantation sites (Figure 2A) were counted, and the respective implantation rate (Figure 2B) was compared. In mice treated with BPA at 200 mg/kg/day, implantation sites were observed in all 14 BPA-treated pregnant mice, and the number of implantation sites did not show significant difference from the control group. In the group treated with BPA (400 mg/kg/day), BPA remarkably reduced the number of implantation sites of pregnant mice. Five of the 10 pregnant mice treated with BPA at 400 mg/kg/day had no implantation sites detected. No implantation sites were found in the pregnant mice treated with BPA at the 600 mg/kg/day and the 800 mg/kg/day groups. These results suggested that BPA with a concentration greater than 400 mg/kg/day reduced the number of implantation sites (Figure 2A) and the embryo implantation rate (Figure 2B).
Figure 1.

Staining of the implantation sites of mice on Day 4.5 of pregnancy after treatment with BPA. (A) The control group (0 mg/kg/day BPA), (B) 200 mg/kg/day BPA group, (C) 400 mg/kg/day BPA group, (D) 600 mg/kg/day BPA group, and (E) 800 mg/kg/day BPA group. Arrow points at the stained blue belt were implantation sites.
Figure 2.
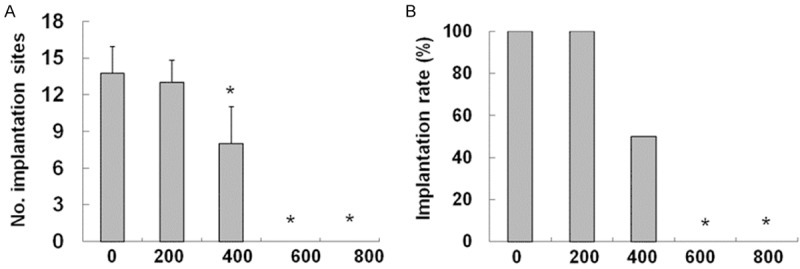
Effects of BPA on the implantation site numbers and implantation rates. A. Effect of BPA on the amount of implantation sites of mice on Day 4.5 of pregnancy. The number of blue belts on the uteri of mice in the control group and treatment groups was counted as the number of implantation sites. N = 10 to 14. The number of implantation sites at each dose was averaged. Error bars: standard deviation. *P < 0.05, compared with the control group. B. Effect of BPA on the implantation rate of mice on Day 4.5 of pregnancy. Implantation rate = total number of mice with implantation sites/total number of mice with fertilized embryos in the genital tract × 100, N = 10 to 15. *P < 0.05, compared with the control group.
BPA delays transfer of embryos from the fallopian tube to uterus
Given that BPA possibly influences embryo development in the genital tract of pregnant mice, we detected the blastocyst development on D4 before the implantation. Compared with the control group, the numbers of embryos washed out from the uterus of pregnant mice treated with BPA at 400, 600, and 800 mg/kg/day were reduced (Figure 3), and embryos were detected in the fallopian tube of these pregnant mice. This result indicated that BPA inhibited the transfer of some embryos from the fallopian tube to the uterus.
Figure 3.
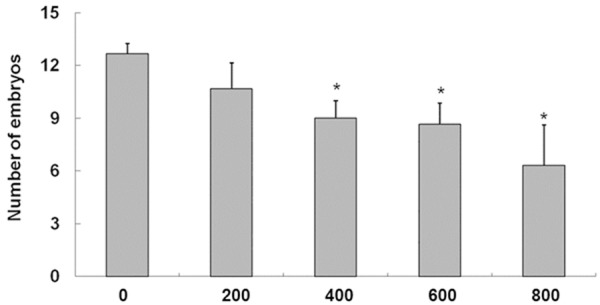
Effect of BPA on the number of embryos in the uteri of mice on D4 of pregnancy. The embryos washed out from the uteri of mice on D4 of gestation were counted. N = 7 to 9. *P < 0.05, compared with the control group.
BPA reduces the blastocyst development and hatching
To determine the effect of BPA on blastocyst development in uteri, the embryos that were washed out from the uteri of pregnant mice treated with BPA on Day 4 of pregnancy were observed (Figure 4A-D). As shown in Figure 4A-E, the embryos with blastocyst cavity were considered as blastocysts. The blastocysts without zona pellucida were hatched blastocysts.
Figure 4.
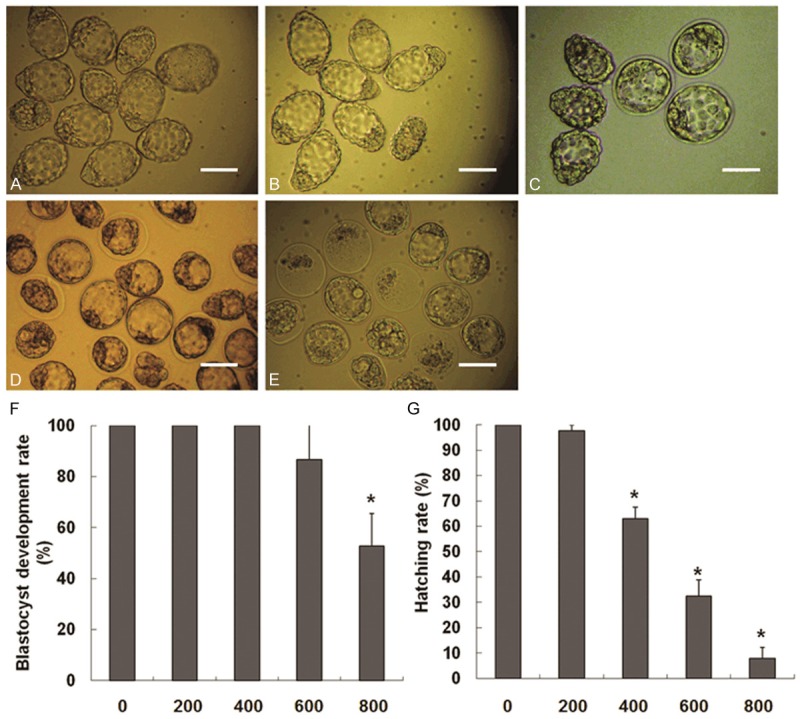
Effect of BPA on the blastocyst development and hatching on D4 of pregnancy. (A) The control group, (B) 200 mg/kg/day BPA group, (C) 400 mg/kg/day BPA group, (D) 600 mg/kg/day BPA group, and (E) 800 mg/kg/day BPA group. Scale = 100 μm. (F) Blastocyst development rate = number of blastocysts/total number of embryos from the uterus on D4 × 100. (G) Hatching rate = number of hatched blastocysts/total number of blastocysts from the uterus on D4 × 100. Number of embryos: N = 43 to 64.
The blastocyst development rate and hatching rate of the embryos washed out from the uteri were determined. It was found that when treated with BPA at 400 and 600 mg/kg/day, the blastocyst development rate did not show significant difference from the control group. In the 800 mg/kg/day BPA group, BPA greatly reduced the blastocyst development rate (Figure 4F) and hatching rate (Figure 4G) significantly when compared with the control group. These results suggested that BPA (800 mg/kg/day) inhibited the hatching of blastocysts. This is a possible reason why blastocysts cannot interact with the endometrium at the implantation stage.
To study the effect of BPA on the quality of hatched blastocysts, the hatched blastocysts were stained for embryonic cell nuclei and apoptotic cells. Hoechst33342 was used in the staining of embryo cell nuclei, and the total cell number of hatched blastocysts was identified by counting the number of embryonic cell nuclei. The total cells of embryos in each group were counted and averaged. Active caspase-3 antibody was used in the staining of apoptotic embryonic cells, and those that showed positive were designated as apoptotic cells. The apoptotic cells in embryos of each group were counted and averaged. The apoptotic cell number of hatched blastocyst in groups at 400, 600, and 800 mg/kg/day of BPA greatly increased compared with the control group (Figure 5A). These results suggested that in groups at 400, 600, and 800 mg/kg/day of BPA, even if some blastocysts could hatch, the quality of hatched blastocysts was decreased, thus reducing implantation of blastocysts. The total cell number of hatched blastocysts in all groups did not show obvious significance (Table 1).
Figure 5.
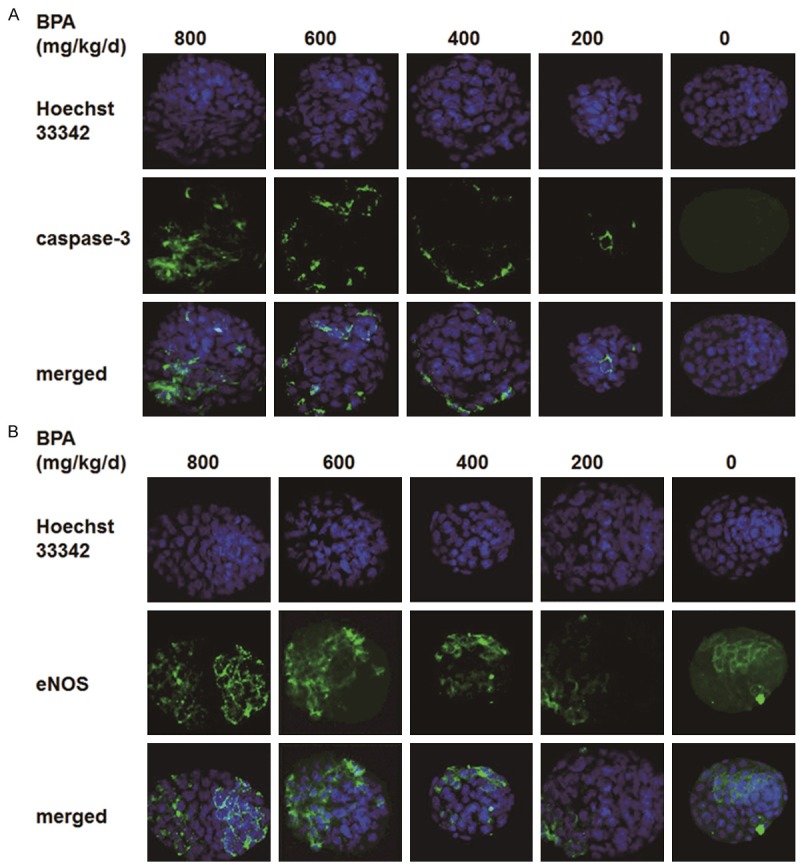
Immunofluorescence staining of hatched blastocyst cells. Embryos were fixed in 4% paraformaldehyde in PBS for 40 min at room temperature, and then permeabilized with 0.2% Triton X-100 in PBS for 10 min. Embryos in the control group, the 200 mg/kg/day BPA group, the 400 mg/kg/day BPA group, the 600 mg/kg/day BPA group, and the 800 mg/kg/day BPA group were stained by incubating the fixed samples with primary antibodies for 2 h, followed by secondary antibodies conjugated with FITC (green) for 60 min. DNA was stained blue by Hoechst33342. Fluorescence was detected on a laser-scanning confocal microscopy (FV-500, Olympus, Japan). All images stained with the same antibody were taken at the same laser power (800×). Merged images were also given. A. Apoptotic embryonic cells were stained green with caspase-3 antibody. B. Embryonic cells with the expressions of eNOS were stained green.
Table 1.
Effects of BPA on the number of total cells and apoptotic cells of hatched blastocysts
| BPA treatment groups | Number of hatched blastocysts | Number of total cells | Number of apoptotic cells |
|---|---|---|---|
| Control group | 13 | 67.33±3.2 | 0.00±0.0 |
| 200 mg/kg/day group | 11 | 61.33±3.2 | 3.00±1.0 |
| 400 mg/kg/day group | 16 | 63±6.0 | 7.33±1.5* |
| 600 mg/kg/day group | 13 | 60.33±9.1 | 13.33±3.1* |
| 800 mg/kg/day group | 18 | 59.67±7.8 | 14.33±3.2* |
P<0.05, compared with the control group.
Effect of BPA on the expression of eNOS in D4 blastocysts
To explore the effect of BPA on the NO metabolism ability of embryonic cells, we detected the eNOS expressions in BPA-treated hatched blastocysts or non-treated hatched blastocysts. The results are shown in Figure 5B. eNOS expression was determined in the cytoplasts of trophoblast cells of hatched blastocysts, but was confined to a minimal number of trophoblast cells. At 200 mg/kg/day, BPA did not exert too much influence on the expression of eNOS in trophoblast cells. Above 400 mg/kg/day, more trophoblast cells were found with the eNOS expression, and the fluorescence staining intensity of eNOS was gradually enhanced (Figure 5B). Moreover, at 800 mg/kg/day, eNOS expression was not only found in the cytoplasts of trophoblast cells but also in the cytoplasts of inner cell mass cells (Figure 5B). These results suggested that BPA increased expression levels of eNOS in the hatched blastocysts. Thus, the NO metabolism ability of hatched blastocysts could be increased.
Discussion
Studies have indicated that BPA influences embryo implantation in the uterus [18-20]. However, due to the differences of administration approaches and time, the concentration range of BPA that influenced embryo implantation had certain differences [17]. Our results showed that gavaged BPA (400 mg/kg/day) decreases embryo implantation. No implantation sites of pregnant mice treated by BPA at 600 and 800 mg/kg/day were observed, during which embryo implantation was inhibited. Xiao et al. conducted a study on subcutaneous injection of BPA at 100 mg/kg/day, and found that it inhibited embryo implantation [12], indicating that the administration approach of the subcutaneous injection was more sensitive to the embryo implantation than the oral administration. However, the most common way of human contact with BPA is repeated contact with the inner packaging of some food [21]. Thus, this experiment could better stimulate the adverse effect from the contact of humans with BPA by oral administration. The administration at different times of the pregnancy would also generate different effects. Berger et al. found that on Day 0 or Day 1 of the pregnancy, subcutaneous injection of 10.125 mg/animal of BPA in pregnant mice significantly reduced the number of implantation sites. However, the injection of BPA at the same dose on Day 2 of pregnancy did not greatly influence the number of embryo implantation sites [22], indicating that the mice became more sensitive to the endocrine disrupter on Day 0.5 to Day 1.5 of pregnancy than on Day 2.5 of the pregnancy. It remains to be studied whether the same effect occurs in pregnant mice at different gestational times after the oral administration of BPA.
Takai et al. added 100 μM of BPA in in vitro culture media of mouse 2-cell embryos. After culture for 48 h, the degradation rate of embryos was significantly improved [23]. Lee et al. cultured mouse 2-cell embryos in culture media added with BPA of different concentrations for 72 h. They found that increase with BPA concentration greatly reduced the blastocyst development rate, 10-4 M BPA inhibited blastocyst development, and no embryos developed to blastocysts [24]. Different from in vitro embryos, in vivo embryos are in the internal environments of the fallopian tube and the uterus and are subject to the influence of different kinds of cells in these two environments. If mouse 2-cell embryos are co-cultured with the epithelial cells of endometrium, the toxic effects of BPA on embryo development can be greatly reduced [24]. However, whether the two internal environments would alleviate the effect of BPA on in vivo embryo development remains unclear. In our experiment, at 800 mg/kg/day, BPA greatly reduced the blastocyst development rate and hatching rate. With the dosages of 400 and 600 mg/kg/day BPA, the blastocyst development rate showed no significant differences from that of the control group yet the hatching rate was greatly reduced. At the dosage of 200 mg/kg/day, BPA did not have a significant effect on blastocyst development rate and hatching rate, indicating that the internal environment of the uterus offered a certain range of protection to embryo development. If the concentration of disrupters exceeds the range, adverse effect would be imposed on in vivo embryo development.
Xiao et al. showed that BPA affected the transfer of the embryo from the fallopian tube to the uterus [12]. In our experiment, the number of embryos washed out from the uterus of BPA-treated pregnant mice at the dosages of 400, 600, and 800 mg/kg/day was much reduced compared with the control group. Different numbers of embryos were detected from the fallopian tubes of pregnant mice. BPA treatment was initiated from Day 0.5 of the pregnancy. At this stage, ovulation and fertilization of mice have been accomplished, which will not influence the total number of fertilized embryos. This finding verified that BPA delayed the transfer of some embryos from the fallopian tube to the uterus, and the delay was enhanced as the BPA concentration increased. The delay of BPA on the transfer of embryos toward the uterus may work on the epithelial cells of the fallopian tube through the estrogen-like effects, so the fallopian tube produces the lock-in effect [25,26] which affects the transfer of embryos.
Tsutsui et al. found that BPA formed DNA adducts with DNA in the golden hamster embryonic cells, leading to the occurrence of chromosome heteroploidy [27]. Pfeiffer et al. applied BPA to process V79 cells but found that BPA did not induce DNA rupture [28]. In our experiment, the numbers of total cells and apoptosis cells of hatched blastocysts in different groups were compared. The number of total cells of hatched blastocysts did not show significant differences among groups. However, at 400, 600, and 800 mg/kg/day, the caspase-3-positive cell number of hatched blastocysts in BPA groups was more increased than that of the control group. Caspase-3 protein, as the executor of cell apoptosis, induced cell apoptosis, which may influence the intruding ability of embryos in the endometrium. Jin et al. administered 2 µg/kg of BPA to adult mice by feeding for 14 consecutive days. The apoptotic cell number of testicular germ cells greatly increased and the mRNA level of Fas, FasL, and caspase-3 mRNA were greatly improved compared with the control group, indicating that BPA promoted the apoptosis of germ cells [29].
NO is a gaseous radical and regulates numerous reproductive processes, such as the synthesis of steroid hormone [30], pregnancy [31-34], follicular development [35,36], and lactation [37,38]. It is produced from the process of L-citrulline generation from the oxidizing reaction of L-arginine catalyzed by NOS; it also has an important role of regulation in early embryo development [39-41]. Studies have revealed that exogenous hormones influence the expression of NOS in embryonic cells [14,15]. However, whether BPA influences the expression of NOS in embryonic cells is not clarified. Thus, eNOS expression in BPA-treated hatched blastocysts or healthy hatched blastocysts were detected. Results showed that the number of trophoblast cells with eNOS expression and the intensity of fluorescence staining were gradually enhanced in above 400 mg/kg/day BPA groups. At 800 mg/kg/day, eNOS expressions were found in the cytoplasts of trophoblast cells and inner cell mass cells. BPA improved the expression level of eNOS in hatched blastocysts, which may generate excessive NO. Adequate amount of NO from normal trophoblast cells can promote the approach and attachment of hatched blastocysts toward the endometrium [16]. However, excessive NO production can cause the delayed decidualization of the endometrium stromal cells and the dysfunction of predecidual cells [42], which influence the attachment of hatched blastocysts with the endometrium and its implantation. Chen et al. found that excessive NO improved the expressions of P53 and Bax in ICR mouse 2-cell embryos, which induced the apoptosis and fragmentization of embryonic cells [43]. Meanwhile, in our experiment, with the increase of BPA concentration, the eNOS expression in embryonic cells improved. Furthermore, the amount of apoptotic cells per embryo also increased, which may be triggered by excessive NO. The generation of excessive NO may be attributed as one of the reasons for the failure of embryo implantation.
In summary, our experimental results showed that the high concentration of BPA damaged the blastocyst development before the implantation, increased the amount of apoptotic cell per embryo and eNOS expression, and influenced the intruding ability of embryos in the endometrium, thereby suppressing embryo implantation. Future studies should identify the target molecules directly or indirectly influenced by NO in hatched blastocysts and clarify the effect of target molecules on blastocyst development and the regulatory mechanism of NO on target molecules.
Acknowledgements
This study was supported by the Scientific and Technological Research Project of Jilin Province (20140204033YY), the “Twelfth Five-Year” Scientific and Technological Research Project of the Education Department of Jilin Province (2013, No. 349), and the Natural Science Foundation Project of Shandong Province (ZR2011HL005).
Disclosure of conflict of interest
None.
References
- 1.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 2.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30:2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varayoud J, Ramos JG, Bosquiazzo VL, Muñoz-de-Toro M, Luque EH. Developmental exposure to Bisphenol A impairs the uterine response to ovarian steroids in the adult. Endocrinology. 2008;149:5848–60. doi: 10.1210/en.2008-0651. [DOI] [PubMed] [Google Scholar]
- 5.Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–24. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 6.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–52. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Toyama Y, Yuasa S. Effects of neonatal administration of 17beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19:181–8. doi: 10.1016/j.reprotox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Rivera OE, Varayoud J, Rodríguez HA, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–12. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 9.Brieño-Enríquez MA, Reig-Viader R, Cabero L, Toran N, Martínez F, Roig I, Garcia Caldés M. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod. 2012;18:171–83. doi: 10.1093/molehr/gar074. [DOI] [PubMed] [Google Scholar]
- 10.Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118:400–6. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G. Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol. 2010;39:34–9. doi: 10.1016/j.domaniend.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and utetine receptivity in mice. Reprod Toxicol. 2011;32:434–41. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tranguch S, Steuerwald N, Huet-Hudson YM. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol Reprod. 2003;68:1538–44. doi: 10.1095/biolreprod.102.009282. [DOI] [PubMed] [Google Scholar]
- 14.Nishikimi A, Matsukawa T, Hoshino K, Ikeda S, Kira Y, Sato EF, Inoue M, Yamada M. Localization of nitric oxide synthase activity in unfertilized oocytes and fertilized embryos during preimplantation development in mice. Reproduction. 2001;122:957–63. doi: 10.1530/rep.0.1220957. [DOI] [PubMed] [Google Scholar]
- 15.Gouge RC, Marshburn P, Gordon BE, Nunley W, Huet-Hudson YM. Nitric oxide as a regulator of embryonic development. Biol Reprod. 1998;58:875–9. doi: 10.1095/biolreprod58.4.875. [DOI] [PubMed] [Google Scholar]
- 16.Saxena D, Purohit SB, Kumer GP, Laloraya M. Increased appearance of inducible nitric oxide synthase in the uterus and embryo at implantation. Nitric Oxide. 2000;4:384–91. doi: 10.1006/niox.2000.0286. [DOI] [PubMed] [Google Scholar]
- 17.Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23:138–44. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol. 2010;30:393–400. doi: 10.1016/j.reprotox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Crawford BR, Decatanzaro D. Disruption of blastocyst implantation by triclosan in mice: impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol. 2012;34:607–13. doi: 10.1016/j.reprotox.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120:978–83. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 22.Berger RG, Shaw J, de Catanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17beta-estradiol. Reprod Toxicol. 2008;26:94–9. doi: 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, Taketan Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15:71–4. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Lee YS, Lee HH, Song HY. Human endometrial cell coculture reduces the endocrine disruptor toxicity on mouse embryo development. J Occup Med Toxicol. 2012;7:7. doi: 10.1186/1745-6673-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Jørgensen EB, Korsgaard B, Le Guevel R, Leffers H, McLachlan J, Møller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkeboek NE, Sonnenschein C, Soto AM. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107:89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM. Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect. 2000;108:723–9. doi: 10.1289/ehp.00108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, Yamaguchi F, Barrett JC. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer. 1998;75:290–4. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer E, Rosenberg B, Deuschel S, Metzler M. Interference with microtubules and induction of micronuclei in vitro by various bisphenols. Mutat Res. 1997;390:21–31. doi: 10.1016/s0165-1218(96)00161-9. [DOI] [PubMed] [Google Scholar]
- 29.Jin P, Wang X, Chang F, Bai Y, Li Y, Zhou R, Chen L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J Biomed Res. 2013;27:135–44. doi: 10.7555/JBR.27.20120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams ML, Nock B, Truong R, Cicero TJ. Nitric oxide control of steroidogenesis: endocrine effects of NG-nitro-L-arginine and comparisons to alcohol. Life Sci. 1992;50:PL35–40. doi: 10.1016/0024-3205(92)90384-2. [DOI] [PubMed] [Google Scholar]
- 31.Ivanova AS, Popova IG, Nazarov SB. Effect of antioxidants and nitric oxide on activity of peritoneal macrophages in albino rats during normal pregnancy. Bull Exp Biol Med. 2013;154:581–3. doi: 10.1007/s10517-013-2003-x. [DOI] [PubMed] [Google Scholar]
- 32.Huang LT, Hsieh CS, Chang KA, Tain YL. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci. 2012;13:14606–22. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, Mao X, Huang Z, Wang F, Wu G, Qiao S. Arginine enhances embryo implantation in rats through PI3K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction. 2013;145:1–7. doi: 10.1530/REP-12-0254. [DOI] [PubMed] [Google Scholar]
- 34.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4:3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Dubey PK, Tripathi V, Singh RP, Saikumar G, Nath A, Pratheesh , Gade N, Sharma GT. Expression of nitric oxide synthase isoforms in different stages of buffalo (Bubalus bubalis) ovarian follicles: effect of nitric oxide on in vitro development of preantral follicle. Theriogenology. 2012;77:280–91. doi: 10.1016/j.theriogenology.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Dubey PK, Tripathi V, Singh RP, Sharma GT. Influence of nitric oxide on in vitro growth, survival, steroidogenesis, and apoptosis of follicle stimulating hormone stimulated buffalo (Bubalus bubalis) preantral follicles. J Vet Sci. 2011;12:257–65. doi: 10.4142/jvs.2011.12.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monasterio N, Morales T. Nitric oxide has a role in attenuating the neuroendocrine response to anaphylactoid stress during lactation. Brain Res. 2011;1402:54–66. doi: 10.1016/j.brainres.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 38.Zaragozá R, Bosch A, García C, Sandoval J, Serna E, Torres L, García-Trevijano ER, Viña JR. Nitric oxide triggers mammary gland involution after weaning: remodelling is delayed but not impaired in mice lacking inducible nitric oxide synthase. Biochem J. 2010;428:451–62. doi: 10.1042/BJ20091091. [DOI] [PubMed] [Google Scholar]
- 39.Lee TH, Lee MS, Huang CC, Tsao HM, Lin PM, Ho HN, Shew JY, Yang YS. Nitric oxide modulates mitochondrial activity and apoptosis through protein S-nitrosylation for preimplantation embryo development. J Assist Reprod Genet. 2013;30:1063–72. doi: 10.1007/s10815-013-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Q, Chen XJ, Ding GL, Dong MY, Huang HF. Downregulative effects of nitric oxide on oocyte fertilization and embryo development: possible roles of nitric oxide in the pathogenesis of endometriosis-associated infertility. Cell Physiol Biochem. 2010;26:1023–8. doi: 10.1159/000323977. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz KR, Pires PR, de Bem TH, Adona PR, Leal CL. Consequences of nitric oxide synthase inhibition during bovine oocyte maturation on meiosis andembryo development. Reprod Domest Anim. 2010;45:75–80. doi: 10.1111/j.1439-0531.2008.01242.x. [DOI] [PubMed] [Google Scholar]
- 42.Ota H, Igarashi S, Oyama N, Suzuki Y, Tanaka T. Optimal levels of nitric oxide are crucial for implantation in mice. Reprod Fertil Dev. 1999;11:183–8. doi: 10.1071/rd99044. [DOI] [PubMed] [Google Scholar]
- 43.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75:1163–71. doi: 10.1016/s0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]


