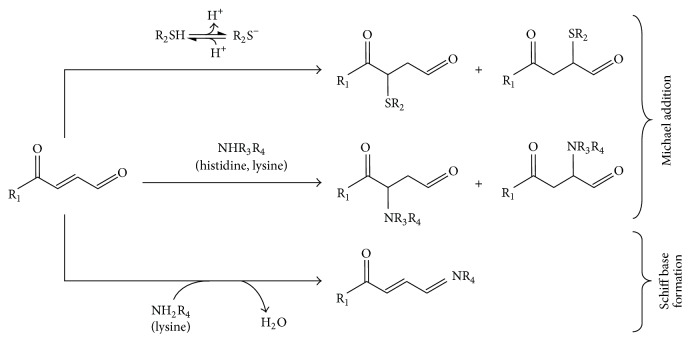
Figure 1.
Reaction scheme of electrophilic lipid derivatives. Electrophilic α,β-unsaturated ketone moieties react with nucleophilic residues on target proteins (thiolates of cysteines and amino groups of histidine and lysine) via Michael reaction. In the case of bifunctional electrophiles, the aldehyde group reacts with primary amines of lysine generating Schiff base adducts.