Table 1. SAR Efforts and Identification of Compound 28.
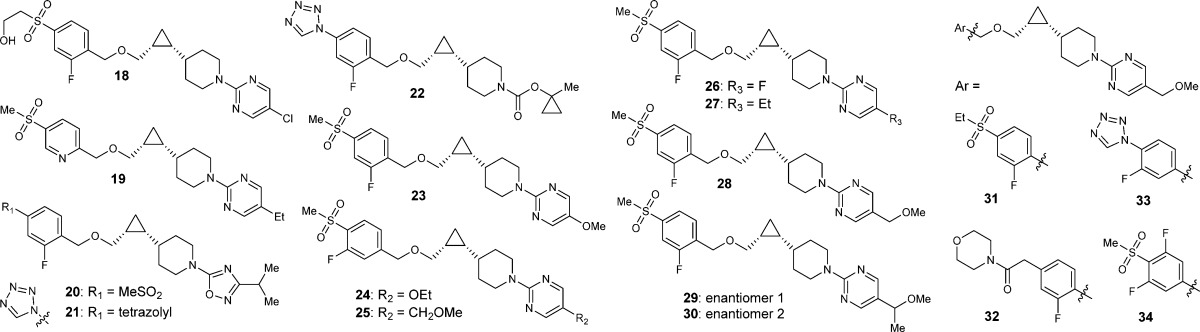
| cmpd | hGPR119a EC50 (nM) | mGPR119a EC50 (nM) | FaSSIFb (μM) | cLogP | HPLC LogD (pH 7) | rat t1/2 (h) | pred. human t1/2 (h)d |
|---|---|---|---|---|---|---|---|
| 2 | 8.7 ± 3.4 (53%) | 3.6 ± 0.1 (56%) | nd | 2.2 | 3.7 | 1.8 | nd |
| 18 | 2.3 ± 0.3 (68%) | 1.7 ± 0.4 (121%) | 83 | 2.9 | 4.8 | 0.9 | 5–7 |
| 19 | 1.5 ± 0.1 (83%) | 2.5 ± 2.1 (153%) | 7 | 2.5 | 3.6 | 1.3 | 8–9 |
| 20 | 4.2 ± 1.0 (87%) | 9.9 ± 5.5 (76%) | 144 | 3.6 | 3.8 | 2.2 | 9–14 |
| 21 | 1.7 ± 0.1 (129%) | 4.8 ± 0.1 (105%) | 103 | 4.1 | 3.9 | 1.4 | 5–9 |
| 22 | 4.8 ± 1.8 (121%) | 15 ± 0.3 (108%) | 127 | 3.6 | 3.8 | 1.0 | 6–7 |
| 23 | 4.5 ± 2.9 (79%) | 1.4 ± 1.2 (85%) | 151 | 3.1 | 4.0 | 0.6 | 4–6 |
| 24 | 1.7 ± 1.1 (75%) | 2.3 ± 0.6 (96%) | 101 | 3.7 | 4.2 | 0.8 | 5–7 |
| 25 | 3.3 ± 1.9 (94%) | 5.2 ± 2.1 (87%) | 155 | 2.4 | 3.5 | 1.2c | 8–9 |
| 26 | 2.4 ± 2.1 (85%) | 4.5 ± 5.6 (131%) | 92 | 2.8 | 4.2 | 1.5 | 12–13 |
| 27 | 2.5 ± 1.9 (98%) | 5.5 ± 2.7 (115%) | 140 | 3.6 | 4.3 | 1.0c | 13 |
| 28 | 2.1 ± 1.3 (88%) | 1.9 ± 0.8 (97%) | 161 | 2.4 | 3.5 | 1.0 | 26 |
| 29 | 3.1 ± 1.2 (87%) | 8.9 ± 9.5 (109%) | 143 | 2.7 | 3.9 | 1.4 | 26 |
| 30 | 4.6 ± 5.0 (95%) | 3.3 ± 1.6 (70%) | 137 | 2.7 | 3.9 | 1.0c | 21 |
| 31 | 7.1 ± 1.4 (58%) | 12.5 ± 0.8 (87%) | 129 | 2.9 | 3.8 | 2.1 | 21 |
| 32 | 3.2 ± 0.6 (104%) | 3.7 ± 0.8 (99%) | 172 | 3.0 | 3.4 | 0.4 | 2 |
| 33 | 1.1 ± 0.3 (84%) | 3.8 ± 1.0 (75%) | 133 | 2.9 | 3.7 | 3.0c | 38 |
| 34 | 4.5 ± 1.9 (80%) | 5.8 ± 3.3 (67%) | 115 | 2.6 | 3.6 | 0.5 | 4 |
Human and mouse GPR119 EC50 data expressed as mean ± SD (n ≥ 2 independent experiments). The percentage in parentheses, % control at max dose: magnitude of cAMP stimulation expressed in % compared to an internal agonist control; the control was defined to have 100% cAMP stimulation.
Kinetic solubility in fasted-state simulated intestinal fluid (FaSSIF) at pH 6.5; in parentheses are cLogP values.
Effective t1/2 calculated from MRT × 0.693 (for compounds exhibiting biphasic PK).
Predicted solely based on rat PK. Where there is only one number (compounds 27–34), allometry was not used since rat Clint was much higher than human. Also, a predicted human half-life in the range of 8–48 h was considered acceptable for further profiling and could potentially support once daily dosing.
