Abstract
The Orphan Drug Act has been successful in providing incentives to find cures for orphan diseases. However, many orphan diseases are still without cure. Therefore, the 114th Congress has introduced the 21st Century Cures Act and the Orphan Product Extension Now Accelerating Cures and Treatment Act of 2015 to further provide incentives to innovators to repurpose existing drugs for treatment of these orphan diseases. However, these bills are currently pending and their incentives might not go far enough.
Background
It is estimated that over 30 million Americans suffer from over 7000 rare diseases or conditions (“orphan diseases”). To provide incentives for innovators to devote research capabilities to find cures for these orphan diseases, President Reagan signed the Orphan Drug Act (ODA) into law in 1983. The ODA provides for the granting of a special status, a so-called “orphan drug designation,” to a product treating a rare disease or condition, upon a sponsor’s request. Under the ODA, a “rare disease or condition” is one that affects (1) less than 200,000 persons in the U.S. or (2) more than 200,000 persons in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for treating such disease or condition will be recovered from the drug’s sales in the U.S. (based on the facts and circumstances as of the date of the request for designation).1 Orphan designation qualifies the sponsor for, among other incentives, a period of seven year market exclusivity from the date of marking approval, presuming the sponsor is the first to obtain such approval from the U.S. Food and Drug Administration (FDA) of the designated drug. During the market exclusivity period, the FDA will not approve another drug for the same indication unless another product is demonstrated to be “clinically superior,” as defined by FDA regulation.
The ODA has been successful. Since the passage of the act and until the end of 2014, the FDA has approved 511 orphan drugs. Some of these orphan drugs were approved for more than a single indication, resulting in multiple seven year periods of market exclusivity. In comparison, prior to the ODA’s enactment in 1983, only 38 drugs were approved for the treatment of orphan diseases.
Current Proposals: The Orphan Product Extension Now Provision of the 21st Century Cures Act and the OPEN ACT
Despite the ODA’s success, cures for many orphan diseases have yet to be found. There are currently several bills to further incentivize drug companies to find such cures. These bills are the 21st Century Cures Act (CCA) (H.R. 6) and Orphan Product Extension Now Accelerating Cures and Treatment Act of 2015 (The OPEN ACT) (H.R. 971 and S. 1421), and each provides incentives to repurpose existing drugs to treat orphan diseases. Both bills provide for what may be best described as an “orphan indication.” Whereas the CCA is an extensive omnibus bill with many different provisions including the “Orphan Product Extension Now” (OPEN provision) (H.R.6 at Subtitle I), the OPEN ACT is focused on finding treatments for rare diseases.
Under the CCA’s OPEN provision, existing pharmaceutical products would receive a one-time, additional six months of exclusivity for an already-approved drug if the drug’s sponsor obtains approval of a new indication for the drug for “a rare disease or condition” (as defined by the ODA). The CCA’s definition of drug includes active compounds, biologics, and orphan drugs. Specifically, the CCA provides for an additional six months of data and market exclusivity for active compounds, biologics, and orphan drugs. Moreover, the CCA provides that for an Orange Book listed patent, a generic will not be approved until six months after the expiration of the patent term (including any patent term extension) unless of course the patent is found to be invalid or not infringed as part of an abbreviated new drug application process and litigation. The additional six-months of exclusivity would be added to the term of exclusivity for the original indication and is in addition to any pediatric and qualified infectious disease exclusivities to which the drug may be entitled. Thus, the CCA provides an incentive for seeking approval for a rare disease or condition (i.e., orphan disease) by providing for extension of exclusivity for the initial indication.
The OPEN ACT is similar to the CCA’s OPEN provision. In fact, for the most part the CCA’s OPEN provision and OPEN ACT even use similar wording. The Senate version of OPEN ACT is almost identical to the CCA’s OPEN provision. Like the CCA, the OPEN ACT provides for a one-time, additional six months of exclusivity for an already-approved drug if the drug’s sponsor obtains approval of a new indication for the drug for a “rare disease or condition” (as defined by the ODA). Similarly, the OPEN ACT would provide for an additional six months of exclusivity for active compounds, biologics, and orphan drugs including the various exclusivities provided for under both the Food, Drug, and Cosmetic Act and the Public Health Service Act, including the 5-year New Chemical Entity exclusivity, 3-year New Clinical Investigation exclusivity, 7-year Orphan Drug exclusivity, and 12-year Reference Product exclusivity for biological products. Again, like the CCA, the OPEN ACT provides an incentive to the innovator by providing an additional six months of exclusivity for the initial indication in return for obtaining approval for treatment of a rare disease or condition.
The House version of the OPEN ACT differs from the CCA’s OPEN provision and the Senate version of the OPEN ACT in that it requires adoptions of final regulations implementing the act within two years after enactment. Moreover, whereas the CCA’s OPEN provision and the Senate version of the OPEN ACT provide for a de facto extension of patent term for any Orange Book listed patent only, the House version of the OPEN ACT includes a similar provision for biosimilar biological products. Specifically, the House version of the OPEN ACT provides that for Orange Book or Purple Book listed patents a generic or biosimilar version will not be approved until six months after the expiration of the patent term (including any patent term extension) unless the patent is found to be invalid or not infringed as part of abbreviated new drug application process or biosimilar application process and litigation.
Will the Incentive Work?
The proposed mechanism for stimulating research into the treatment of orphan diseases in the CCA and the OPEN ACT closely mimics the framework of the Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA), both of which provide for incentives to pursue pediatric indications. The BPCA and PREA were born out of observed differences in responses to drugs between children and adults, including different dosages, unique pediatric adverse events, and even the inability to demonstrate effectiveness.2 Because of these differences, approximately 65% to 80% of drugs have not been tested in children.2 The PREA and BPCA work in conjunction to provide an additional six months of patent exclusivity and market exclusivity if a drug is shown to be safe in children. According to the Pharmaceutical Research and Manufacturers of America (PhRMA), the PREA has been highly successful: “The combination of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA) has driven research to create innovative medicines for children younger than 18 and has greatly advanced American children’s medical care.”3 Based on the success of the pediatric indications, such a framework may work to incentivize repurposing existing drugs to the treatments for orphan diseases.
Should the OPEN ACT or the CCA’s OPEN provision become law, innovators with an approved drug that is subsequently shown to be effective in the treatment of an orphan disease will have two options for obtaining regulatory approval for the treatment of the orphan disease. The innovators can either pursue the currently existing ODA pathway or the “OPEN” pathway. The ODA pathway gives innovators seven years of market exclusivity for the new orphan disease indication. Alternatively, the innovators can follow the “OPEN” pathway, which gives the innovator an additional six months of exclusivity for the initial indication. These pathways are illustrated in the flowchart below.
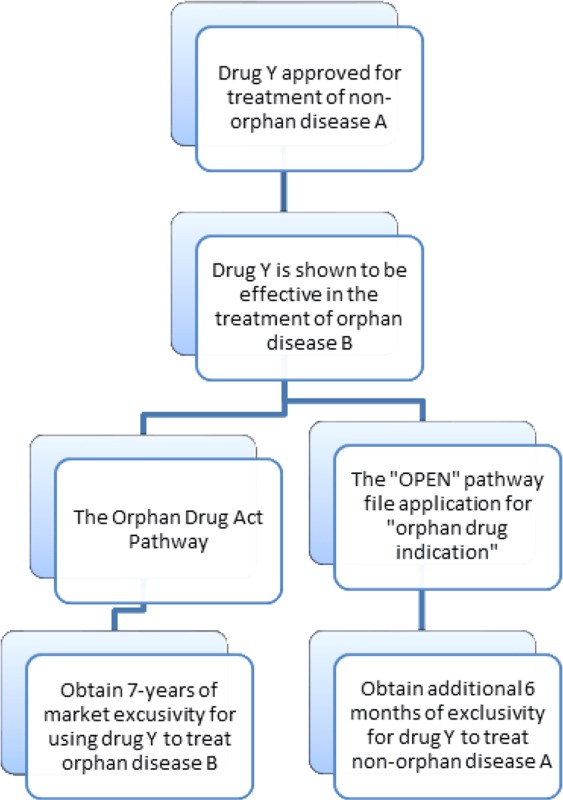
However, whether the OPEN pathway provides enough of an incentive to repurpose an existing drug for the treatment of an orphan disease is highly dependent on the drug.
While there are certain differences in the response to a drug between adults and children, innovators pursuing a pediatric indication for their drugs know that the drug is able to treat the approved disease. As such, in many circumstances, the likelihood of success of showing efficacy in children may be somewhat predictable based on the drug’s effectiveness in adults. The same cannot be said for pursuing an orphan drug indication. While it may be possible to somewhat predict the likelihood of success of a clinical study for an orphan drug indication based on similarities in disease pathways not all orphan disease have a disease pathway that is similar to the pathway of the initial disease for which the drug is approved. It is also difficult to estimate how many of the currently approved drugs will be suitable for orphan disease treatment. In addition, the economic benefit is yet to be determined whether an innovator should to pursue the OPEN pathway or the ODA pathway.
Of the currently pending proposals, the House version of the OPEN ACT appears to provide the most incentive to encourage repurposing of existing drug to treat orphan disease. Specifically, whereas the OPEN provision of the CCA and Senate version of the OPEN ACT provide innovators with the market exclusivities for biologics that it might not receive under patent protection, the House version of the OPEN ACT also provides additional patent exclusivity for biologics. By including the patent exclusivity for biologics, the House version of the OPEN ACT provides encouragement to pursue orphan indications for approved biologics that are protected by patents.
In short, the passage of the CCA or the OPEN ACT will likely provide incentives for innovators to find treatment for orphan diseases. However, depending on the drug and the costs associated with obtaining an orphan drug indication, the six month exclusivity incentive might not be enough.
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- As part of a request for orphan designation of a product to treat a disease or condition that affects more than 200,000 persons, a sponsor would have to submit to FDA, among other things, (1) a statement of and justification for production and marketing costs that the sponsor has incurred in the past and expects to incur during the first seven years that the drug is marketed; and (2) an estimate of and justification for the expected revenues from sales of the drug in the United States during its first seven years of marketing. 37 C.F.R. §§ 316.20, 361.21.
- Thaul, S. FDA’s Authority to Ensure that Drugs Prescribed to Children are Safe and Effective; Congressional Research Service RL33986 (June 25, 2012).
- PhRMA Statement on Tremendous Success of BPCA and PREA (April 28, 2011). http://www.phrma.org/media/releases/phrma-statement-tremendous-success-bpca-prea.


