Figure 2.
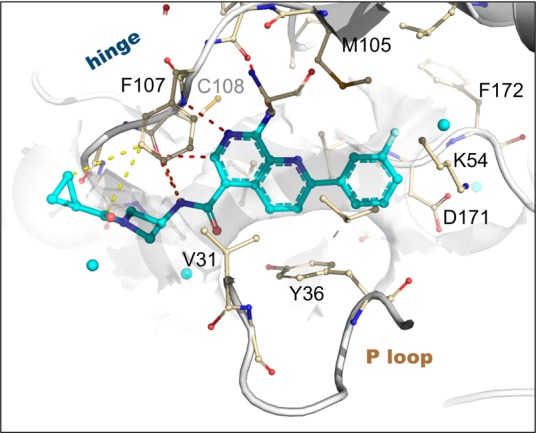
X-ray structure of 13 bound to MAP4K4 at 2.89 Å (PDB: 4ZK5). Select hydrogen bond (red), pi stacking (orange), and van der Waals (yellow) interactions are highlighted. Proximal ordered waters are shown in cyan spheres. The solvent-accessible protein surface in the vicinity of the ligand is shown in white, demonstrating the extensive contribution to the constricted enclosure created by the approach of Tyr36 enabled by a buckled P loop conformation. The ligand emerges toward solvent at the left, where the azetidine moiety creates a bend around the Phe107 side chain to maintain close contacts to the protein surface.
