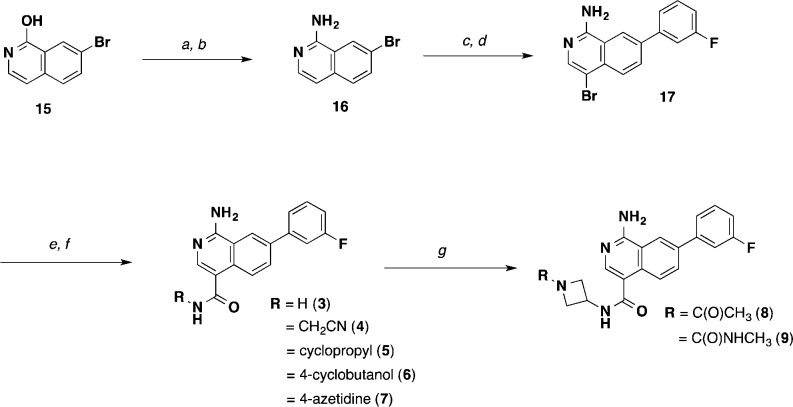
Scheme 1. Synthesis of Isoquinoline MAP4K4 Inhibitors (3–9).
Reagents and conditions: (a) POCl3, 100 °C, 3 h, 80%; (b) aq. NH3, NMP, 148 °C, 89%; (c) 3-fluorophenylboronic acid, Pd(dppf)Cl2, Na2CO3, 1,4-dioxane/H2O, 99%; (d) NBS, DMF, 0 °C, 49%; (e) CO (50 psi), Pd(dppf)Cl2, DMAP, MeOH, 70 °C; NaOH, H2O, MeOH, THF, 44%, 2 steps; (f) RNH2, HATU, DIPEA, DMF or THF; (g) (i) HCl, EtOAc/DCM; (ii) for 8, CH3CO2H, HATU, DIPEA, THF; (iii) for 9, CDI, DIPEA, CH3NH2.