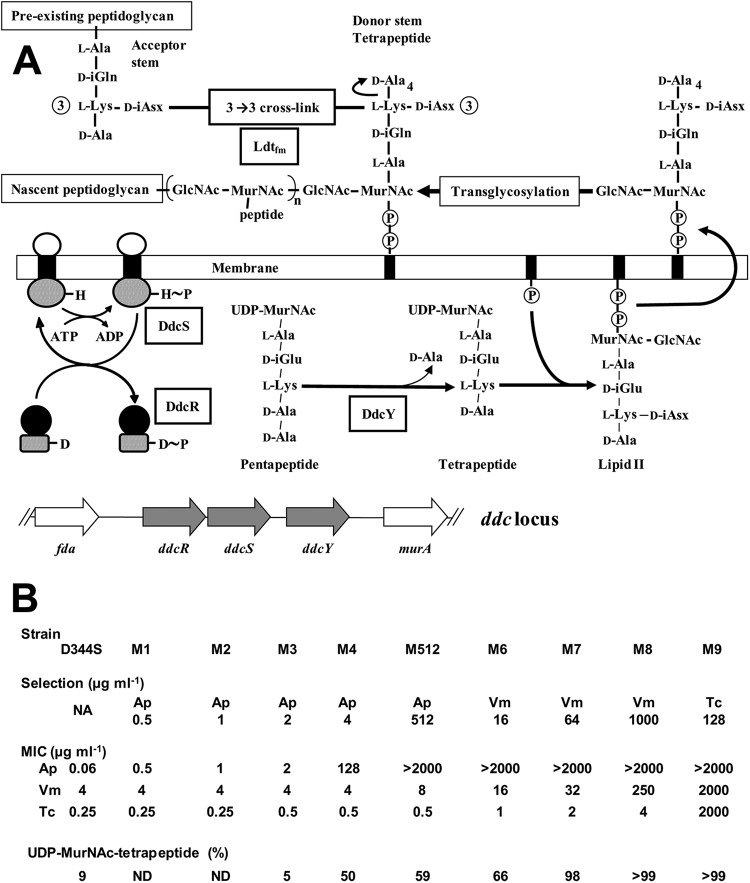
FIG 1.
Activation of the l,d-transpeptidation pathway in response to ampicillin and glycopeptides in Enterococcus faecium. (A) Map of the ddc locus and role of the encoded proteins in peptidoglycan synthesis. The black boxes represent the undecaprenyl lipid transporter inserted into the cytoplasmic membrane. Phosphates are indicated by circled Ps. Transcriptional activation by DdcRS involves autophosphorylation of DdcS on a His residue (H∼P) and subsequent transfer of the phosphate group onto an Asp residue of DdcR (D∼P). Peptidoglycan cross-linking by l,d-transpeptidase Ldtfm involves cleavage of the l-Lys3-d-Ala4 peptide bond of a stem tetrapeptide (acyl donor) and formation of an amide bond between the carbonyl of l-Lys3 and the α-amino group of d-iAsn of the acyl acceptor. (B) Characteristics of the parental strain D344S and mutants M1 to M9. Serial selection was performed with ampicillin (Ap), vancomycin (Vm), or teicoplanin (Tc) at the indicated concentrations. The resulting increases in MICs for the three drugs are indicated. Selection for resistance resulted in increased UDP-MurNAc-tetrapeptide (shown as a percentage of the total cytoplasmic pool of precursors) to the detriment of UDP-MurNAc-pentapeptide following hydrolysis of d-Ala5 by d,d-carboxypeptidase DdcY. NA, not applicable.