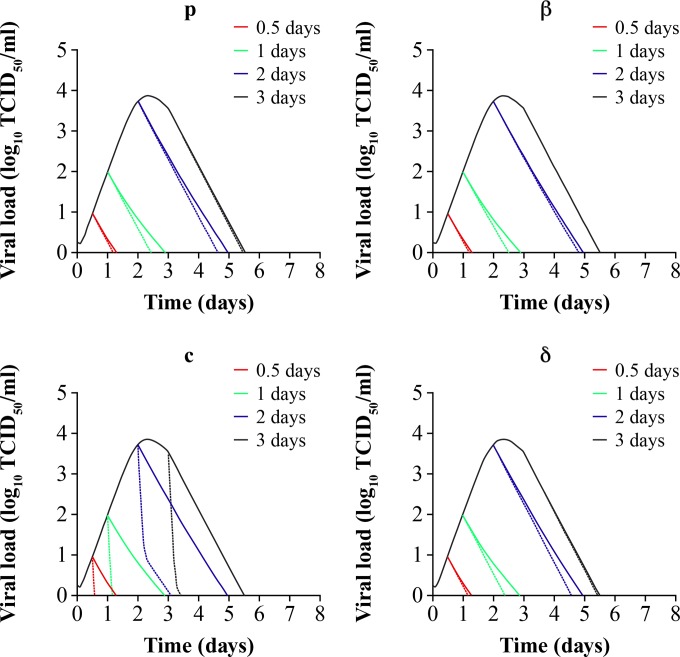
FIG 5.
Simulations predicting the combined in vivo pharmacologic effects of 75-mg b.i.d. oseltamivir standard therapy on influenza viral dynamics, decreasing viral production (p), decreasing infection rate (β), increasing viral clearance rate (c), and increasing clearance rate (δ) of infected cells. Treatment is started at 0.5 (red line), 1 (green line), 2 (blue line), and 3 (black line) days after infection. Solid lines depict the single pharmacologic effect of the standard oseltamivir 75-mg b.i.d. regimen administered over 5 days. Dashed lines show the combined pharmacologic effects. All secondary effects were changed 10-fold from their baseline values in Table 2.