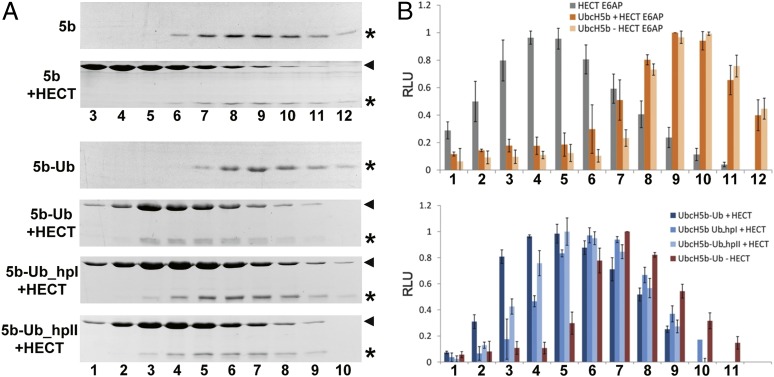
Fig. 2.
The ubiquitin-loading status of UbcH5b affects its ability to interact with E6AP. (A) UbcH5b (5b) and stable conjugates of the catalytically inactive UbcH5b–C85K mutant with wild-type ubiquitin (5b-Ub) or the ubiquitin mutants Ub_hpI and Ub_hpII (5b-Ub_hpI, 5b-Ub_hpII) were incubated in the absence or the presence (+HECT) of the HECT domain of E6AP. After 5 min, the mixtures were fractionated by size exclusion chromatography. Fractions were subjected to SDS/PAGE and proteins visualized by Coomassie staining. Relative fraction numbers are indicated. Running position of the HECT domain is marked by an arrowhead; running positions of UbcH5b and the different UbcH5b–ubiquitin conjugates are marked by an asterisk. (B) Intensities of the bands representing the HECT domain, UbcH5b, and the different UbcH5b–ubiquitin conjugates were quantified by densitometry and are expressed in relative units (RLU). Relative fraction numbers are indicated. Error bars represent the SD from at least three independent experiments. For results obtained for UbcH7–ubiquitin conjugates, see Figs. S1 and S2.