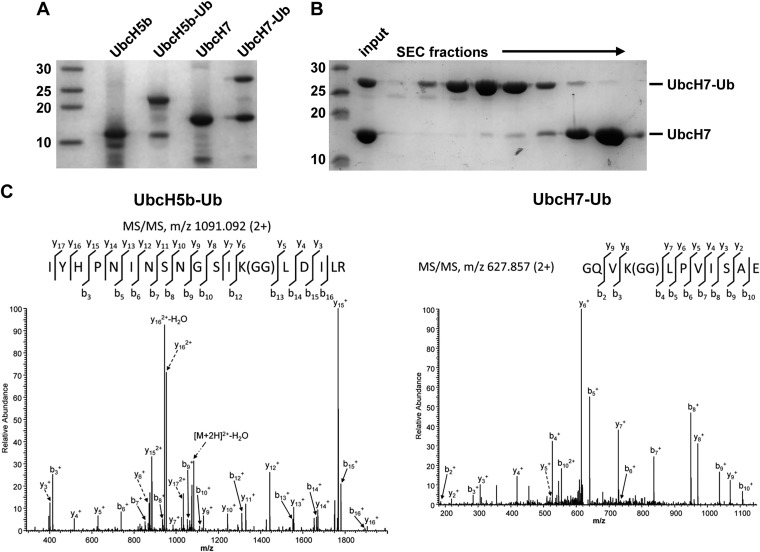
Fig. S1.
Generation of stable conjugates between ubiquitin and UbcH5b and UbcH7. (A) To generate stable conjugates between ubiquitin and UbcH5b or UbcH7 mimicking respective ubiquitin thioester complexes, the catalytic Cys residue of UbcH5b (Cys-85) and of UbcH7 (Cys-86) was substituted by Lys. The respective mutants were incubated with the ubiquitin-activating enzyme and ubiquitin to allow covalent attachment of ubiquitin to Lys-85 of UbcH5b or Lys-86 of UbcH7 via isopeptide bond formation. Whole reaction mixtures were separated by SDS/PAGE with respective input controls (UbcH5b, UbcH7) followed by Coomassie staining. The running positions of molecular mass markers (in kilodaltons) are indicated. (B) For interaction analysis with the HECT domain of E6AP, ubiquitin conjugates of UbcH7 (or UbcH5b) were purified from the nonmodified form by size exclusion chromatography (SEC). Fractions were analyzed by SDS/PAGE followed by Coomassie staining. The running positions of molecular mass markers (in kilodaltons) are indicated. (C) Bands in A representing stable conjugates between ubiquitin and UbcH5b (UbcH5b–Ub) and UbcH7 (UbcH7b–Ub) were cut out, digested by trypsin (5b–Ub) or a trypsin/Glu-C mixture (7-Ub), and desalted. Digested samples were analyzed by reversed phase liquid chromatography-nanospray tandem mass spectrometry (LC-MS/MS) (for details, see SI Materials and Methods). The results obtained showed that UbcH5b and UbcH7 were exclusively modified by ubiquitin at Lys-85 and Lys-86, respectively.