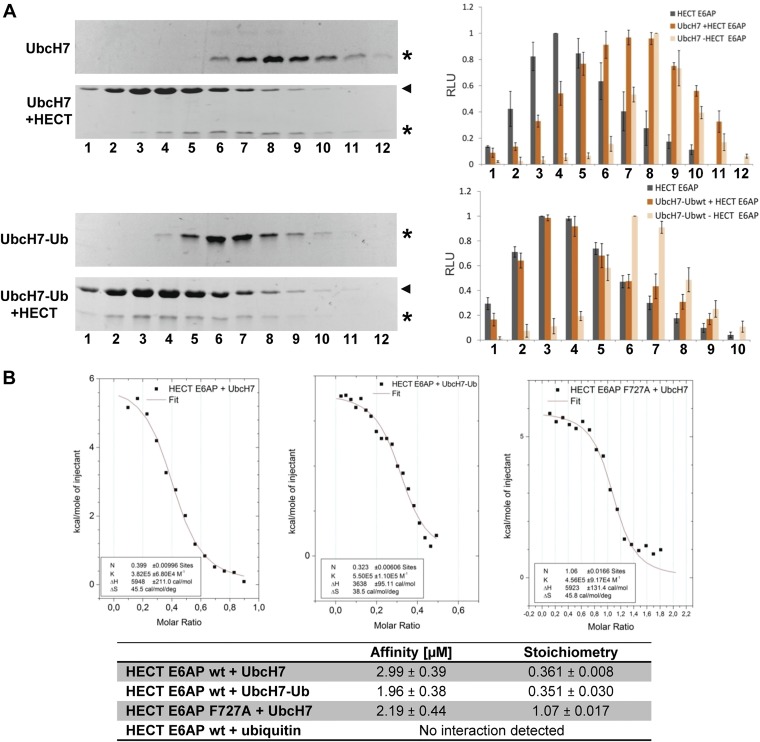
Fig. S2.
The ubiquitin loading status has no major impact on the ability of UbcH7 to interact with E6AP. (A) UbcH7 and the stable UbcH7–ubiquitin conjugate (UbcH7–Ub) were incubated in the absence or in the presence (+HECT) of the HECT domain of E6AP for 5 min. The mixtures were then fractionated by size exclusion chromatography. Fractions were subjected to SDS/PAGE and proteins visualized by Coomassie staining. Intensities of the bands representing the HECT domain, UbcH7, and the different UbcH7–ubiquitin conjugates were quantified by densitometry and are expressed in relative light units (RLU) (Right). Running position of the HECT domain is marked by an arrowhead; running positions of UbcH7 and the different UbcH7–ubiquitin conjugates are marked by an asterisk. Relative fraction numbers are indicated. Error bars represent the SD from at least three independent experiments. (B) The binding affinity of ubiquitin, UbcH7, and the UbcH7–ubiquitin conjugate for the HECT domain of E6AP was determined by isothermal titration calorimetry (for details, see SI Materials and Methods). Origin software was used to fit the data to a single-site binding model and determine the stoichiometry (N), ΔH, ΔS, and the association constant K. Measurements for the interaction of UbcH7 and UbcH7–ubiquitin with the HECT domain of E6AP were done in triplicates. Note that the apparent stoichiometry of UbcH7 to the HECT domain of ∼0.36 is in line with previously published data showing that the HECT domain of E6AP forms trimers at high concentrations and that the trimer is bound to only one UbcH7 molecule (55). Therefore, ITC was repeated with the F727A mutant of the HECT domain (substitution of Phe-727 by Ala), which does not form trimers, revealing that one HECT domain molecule binds to one UbcH7 molecule.