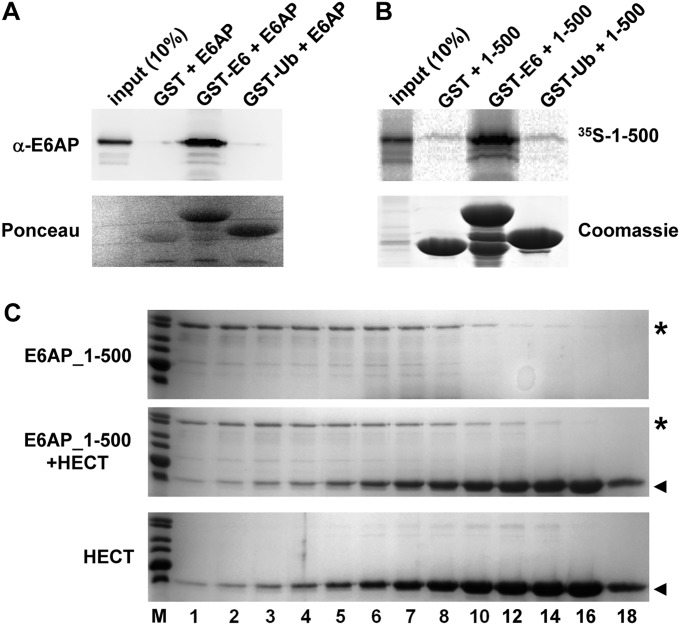
Fig. S6.
Interaction studies. (A) Baculovirus-expressed E6AP was incubated with bacterially expressed GST or GST fusion proteins of HPV16 E6 (GST–E6) and ubiquitin (GST–Ub) for 2 h at 4 °C. GST proteins were pulled down by glutathione-Sepharose beads and precipitates analyzed by SDS/PAGE followed by Western blot analysis using an anti-E6AP antibody or by Ponceau staining. Whereas E6AP is efficiently bound by GST–E6, an interaction with GST–Ub was not observed. (B) A C-terminally truncated form of E6AP comprising amino acids 1–500 was translated in rabbit reticulocyte lysate in the presence of 35S-Met. The translate was incubated with bacterially expressed GST, GST–E6, or GST–Ub as indicated for 2 h at 4 °C. GST proteins were pulled down by glutathione-Sepharose beads and precipitates analyzed by SDS/PAGE followed by fluorography (to detect 35S-labeled 1–500) or by Coomassie staining (to visualize GST proteins). (C) The N-terminal 500 amino acids of E6AP (E6AP_1–500) were expressed as a GST fusion protein in bacteria. Upon purification, E6AP_1–500 (final concentration 10 µM) alone (Upper), E6AP_1–500 (10 µM) and the HECT domain (100 µM) (Middle), and the HECT domain (100 µM) alone (Lower) were incubated for 5 min at room temperature. The mixtures were fractionated by size exclusion chromatography and the fractions were analyzed by SDS/PAGE followed by Coomassie staining. Relative fraction numbers are indicated. Running positions of E6AP_1–500 and the HECT domain are marked by an asterisk and an arrowhead, respectively. Note that the presence of the HECT domain did not affect the elution behavior of E6AP_1–500 and vice versa, indicating that under the conditions used, the two proteins did not detectably interact.