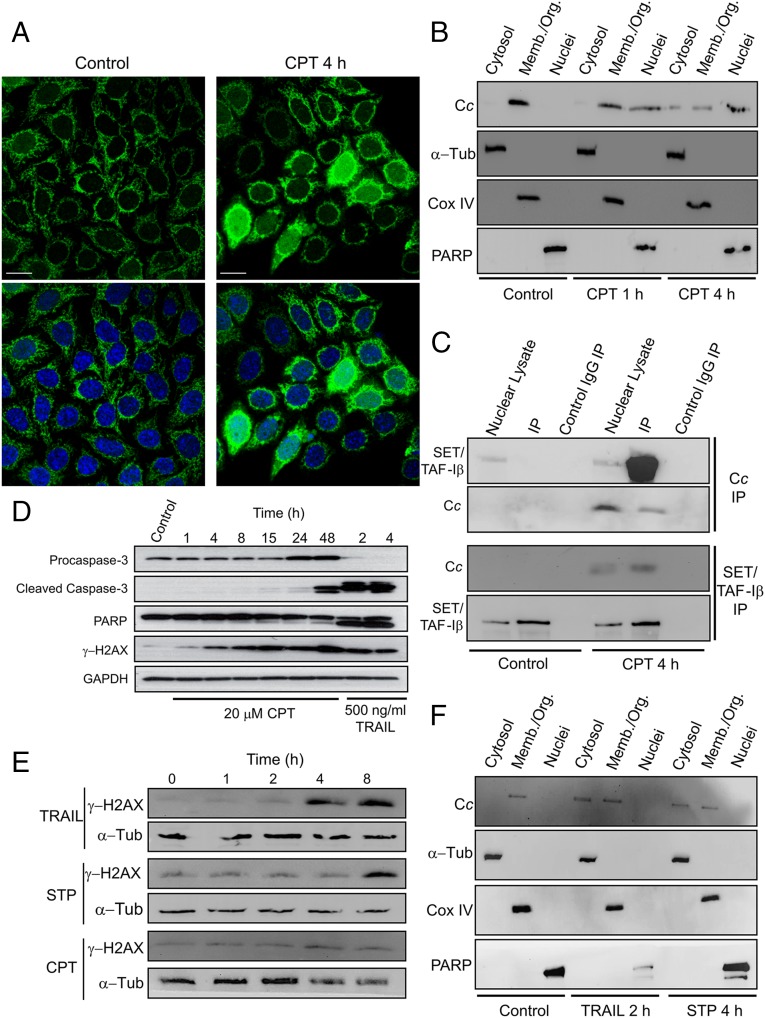
Fig. 1.
CPT-induced nuclear translocation of Cc and formation of the Cc:SET/TAF-Iβ complex. (A) Subcellular localization of Cc-GFP stably expressed (green; Upper) in Heltog cells upon treatment with 20 μM CPT for 4 h detected by confocal microscopy (60x oil objective). Nuclei were stained in blue with Hoechst. Colocalization of green Cc-GFP fluorescence and blue nuclear staining is shown in the merge images (Lower). (Scale bars, 25 μm.) (B) Subcellular fractioning showing endogenous Cc location upon treatment with 20 μM CPT for 1 or 4 h. Nontreated and CPT-treated Heltog cells were fractionated to yield cytosolic, membrane/organelle (Memb./Org.) and nuclear fractions. Purity of subcellular fractions was verified by Western blot using anti–α-Tub (50 kDa), anti-Cox IV (17 kDa), and anti-PARP (116 kDa) antibodies. (C, Upper) IP of SET/TAF-Iβ with endogenous Cc after treating Heltog cells with 20 μM CPT for 4 h. Western blot showed the detection of SET/TAF-Iβ as an ∼34-kDa band (lanes 1 and 4) in the nuclear fraction. Cc-IP of nuclear lysates from nontreated (lane 2) and CPT-treated cells (lane 5), followed by probing with the SET/TAF-Iβ antibody. Confirmation of immunoprecipitated Cc from nuclear lysates is also shown (lanes 4 and 5) under CPT treatment. (Lower) Reverse IP of endogenous Cc with SET/TAF-Iβ following CPT treatment. Detection of Cc as an ∼12-kDa band in the nuclear lysate (lane 4) and in the IP of SET/TAF-Iβ of CPT-treated cells (lane 5). Mouse IgG was used as control (lanes 3 and 6). (D) Detection of caspase-3 activation in Heltog cells treated with 20 μM CPT for 1, 4, and 24 h and 100 ng/mL TRAIL for 2 h. Cleaved caspase-3 (19 and 17 kDa) and full-length (116 kDa) or cleaved PARP (89 kDa) was detected by Western blot. Procaspase-3 (32 kDa) and α-Tub (50 kDa) antibodies were used as controls. (E) DNA-damage response upon treatment of Heltog cell cultures with 100 ng/mL TRAIL and 1 μM STP or 20 μM CPT for 0, 1, 2, 4, and 8 h. Specific antibody against phosphorylated γ-H2AX was used in Western blotting. α-Tub antibody was used as loading control. (F) Subcellular fractioning showing endogenous Cc location upon treatment with 100 ng/mL TRAIL for 2 h or 1 μM STP for 4 h.