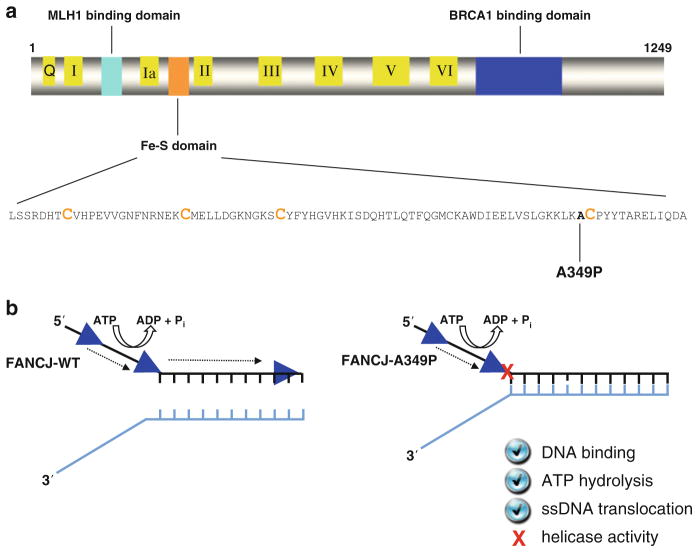
Fig. 6.4.
An FA complementation group J patient mutation (A349P) in the conserved Fe-S domain uncouples DNA ATPase and translocase activities from strand separation (helicase) activity. (a) FANCJ protein with the conserved helicase core domain, key protein interaction domains, and the Fe-S cluster. The conserved helicase motifs are indicated by yellow boxes, and the protein interaction domains for MLH1 and BRCA1 are shown by aqua green and blue boxes, respectively. The expanded Fe-S domain shows the locations for conserved cysteine residues in orange, and the A349P missense mutation of a FANCJ patient in bold. (b) The purified recombinant FANCJ-A349P protein fails to couple ATPase and single-stranded DNA translocase activity to unwinding duplex DNA. See text and ref. [59] for details