Figure 7.
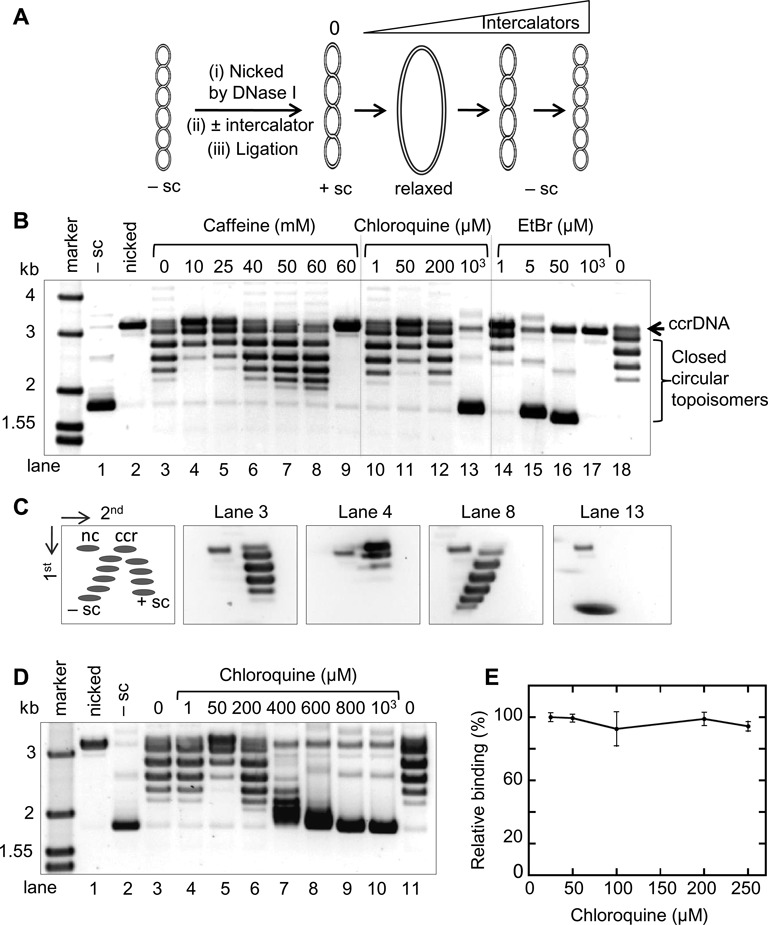
The dsDNA intercalating activity of caffeine does not affect its inhibition of Rad51 binding to ssDNA. (A) Diagram representation of an intercalating assay. Negatively supercoiled plasmid was first nicked, then incubate with or without intercalator. The nick was subsequently sealed by ligation to fix any topological changes induced by the intercalator. The resulting topoisomers can be analyzed by agarose gel electrophoresis. Upon binding intercalator, the nicked plasmid unwinds toward the relaxed state, then to the negative supercoiling (–sc) states as the concentration of intercalator increases. (B) The effect of caffeine, chloroquine and ethidium bromide on the conformation of dsDNA. Negatively supercoiled plasmid pBlueScript was nicked and religated in the presence of different concentrations of intercalating compounds (refer to diagram in (A)). The reaction products containing topoisomers were separated by electrophoresis on 1% agarose gel. Lanes 1, 2 and 9 have no ligase. ccrDNA: covalently closed, relaxed DNA. (C) 2D agarose gel analysis of topoisomers. Schematic diagram of the relative positions of topoisomers resolved on 2D gels is shown; nc, nicked, circular DNA; ccr, covalently closed, relaxed DNA; −sc, negatively supercoiled DNA; +sc, positively supercoiled DNA. Selected samples from reactions in section (B) above (lanes 3, 4, 8 and 13) were analyzed. (D) The concentration effect of chloroquine on dsDNA. The assay was carried out as in section (B) above by titrating chloroquine concentration to obtain an intercalating effect similar to that observed for 60 mM caffeine. (E) ScRad51 binding to ssDNA assayed by fluorescence polarization in the presence of increasing concentrations of chloroquine. Rad51 was 150 nM, ssDNA was fluorescein-tagged 84 mer (200 nM nucleotides or 2.4 nM). The binding reaction was at 37°C for 30 min. Error bars represent standard deviation from triplicate trials.
