Figure 3.
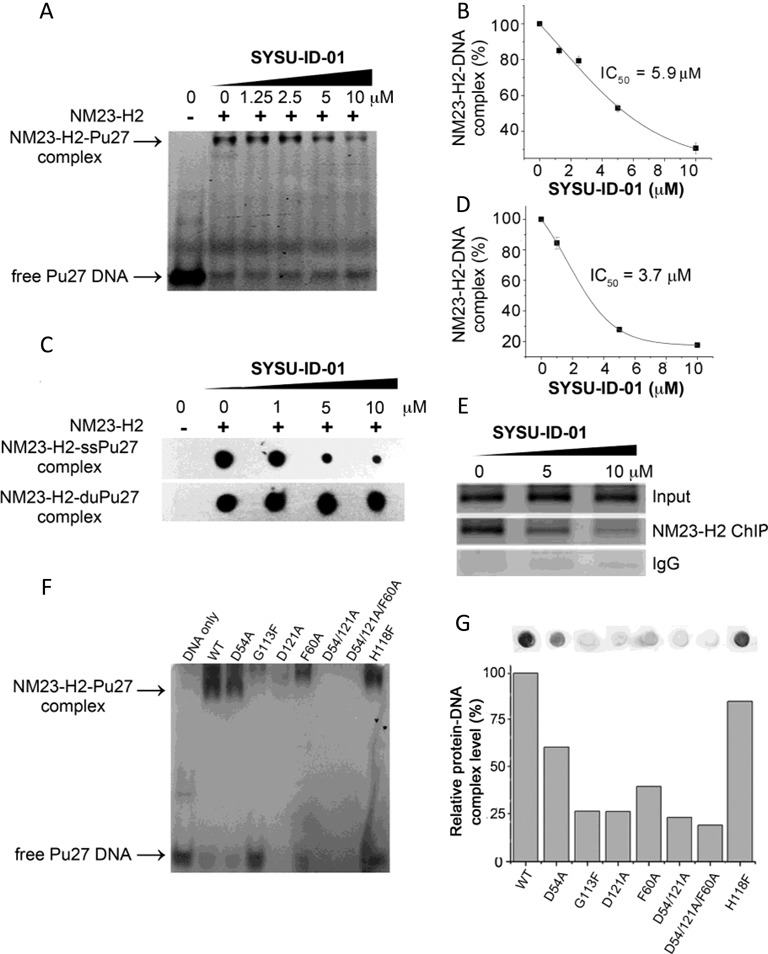
Disruption of the protein–DNA interaction by SYSU-ID-01. (A) Electrophoretic mobility shift of 5′-FAM-labelled Pu27 from c-MYC promoter in presence or absence of NM23-H2 and/or SYSU-ID-01 in 8% native PAGE. Top panel: NM23-H2–DNA complex, bottom panel: free Pu27 DNA. (B) Dose-dependent inhibition curve for NM23-H2 binding to Pu27 in the presence of increasing concentrations of SYSU-ID-01 obtained from a gel mobility shift assay. The data were derived from three experiments and were shown as the means ± S.E.M. (C) Filter-binding assay results reflected the effect of SYSU-ID-01 on the combination of NM23-H2 with the c-MYC G-rich sequence Pu27 (SS-Pu27) and double-stranded c-MYC Pu27 (DS-Pu27). (D) Dose-dependent inhibition curve for NM23-H2 binding to SS-Pu27 in the presence of increasing concentrations of SYSU-ID-01 obtained from a filter-binding assay. The data were derived from three experiments and were shown as the means ± S.E.M. (E) ChIP assays to evaluate the disruption of NM23-H2–DNA interaction by SYSU-ID-01 in the HeLa cell line. Normal rabbit IgG was used as the negative control for mock immunoprecipitation. Immunoprecipitated DNA samples were PCR-amplified to show NM23-H2 occupancy of the c-MYC promoter, the amplified products were separated on a 1.5% agarose gel. (F) Gel mobility shift results of wild-type and mutant NM23-H2 binding to Pu27. (G) Filter-binding assay results of wild-type and mutant NM23-H2 binding to Pu27 in the graph and quantitative columns. (A) and (F) had 60 pmol Pu27 in each sample, while the DNA amount was 100 fmol in (C) and (G) samples. SYSU-ID-01 treatments were for 1 h at 25°C before detection.
