Figure 8.
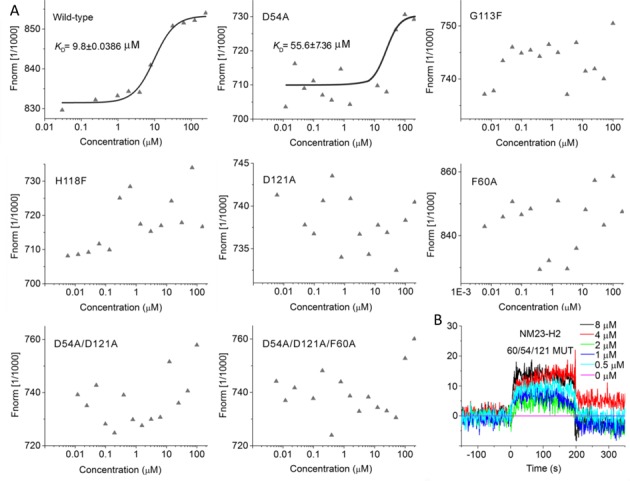
Binding affinity of wild-type and mutant NM23-H2 to SYSU-ID-01. (A) The MST results provided us with a quantitative impression of the interaction of wild-type and mutant NM23-H2 to SYSU-ID-01. Only wild-type NM23-H2 gives a typical binding curve and leads to a KD = 9.8 ± 0.0386 μM, while the data points for mutant NM23-H2 proteins were chaotic, which demonstrated the weaker binding affinities. The SYSU-ID-01 was titrated from 6 nM to 200 μM, the concentration of NT-647 labelled NM23-H2 was kept constant at 200 nM. (B) SPR sensorgrams for the binding of SYSU-ID-01 to immobilized D54A/D121A/F60A mutant NM23-H2 in SPR assays, the ligand concentrations in the flow solutions were 0–8 μM. The sensorgrams of SYSU-ID-01 binding to D54A/D121A/F60A mutant NM23-H2 were comparatively low and chaotic, which revealed the weaker combining capacity.
