Figure 4.
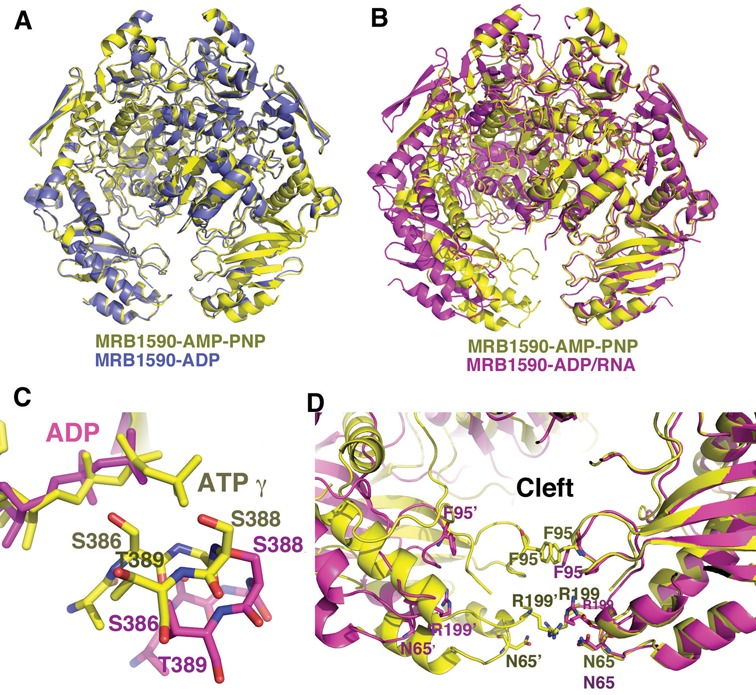
MRB1590 adopts open and closed states. (A) Overlay of the structures of MRB1590–ADP (blue) and MRB1590–AMP–PNP (yellow). The structures, including their dimer conformations are essentially identical. (B) Overlay of one subunit of the MRB1590–AMP–PNP (yellow) dimer onto that of the MRB1590–ADP complex obtained in the presence of RNA (magenta). Here, the subunits are the same but the dimers are dramatically different. The main result being that the N-domains are driven far apart in the RNA bound form, creating an open cleft between the N-domains. (C). Comparison of the location of the signature region in the non-overlaid subunit in the AMP–PNP bound or closed form (yellow) and the open (magenta) state. In the open state, the signature region is translocated too far to interact with the nucleotide that is bound in the other subunit. (D) Comparison of the N-domain cleft in the open and closed states. In the closed state, there are interactions between residues in the subunits of the dimer that block the channel. In particular, the 2-fold related Phe95 side chains and Arg199 side chains stack with each other. Arg199 also hydrogen bonds to Asn65’. These interactions are not present in the open state (magenta), which harbors a wide opening.
