Figure 7.
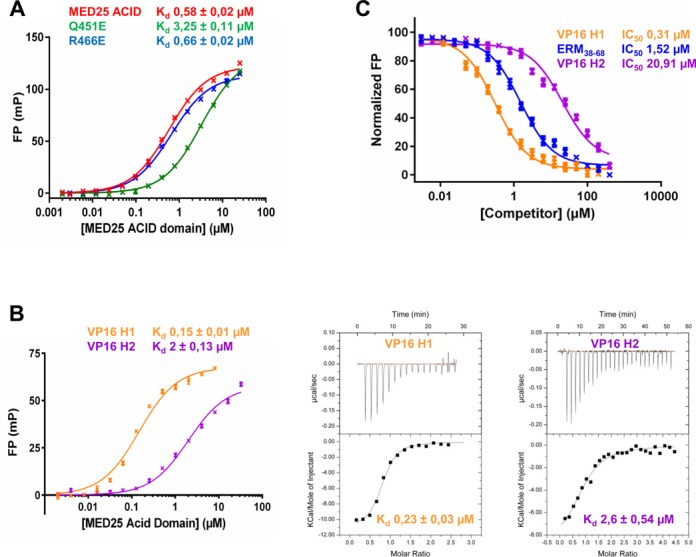
Competition between ERM38–68, VP16 H1 and VP16 H2 for binding to MED25 ACID/PTOV. (A) Comparison of the dissociation constant (Kd) values for the binding of MED25 ACID/PTOV domain and its mutants (Q541E and R466E) to ERM38–68. (B) (left) FP experiments and (right) ITC data of a titration of MED25 ACID/PTOV with VP16 H1 or VP16 H2 peptides to measure the binding affinity. (C) IC50 values for the displacement of TAMRA-ERM38–68 (4 nM) from MED25 ACID/PTOV (0.2 μM) by unlabelled ERM38–68 (LogEC50 = 0.18 ± 0.03), VP16 H1 (LogEC50 = −0.51 ± 0.04) and VP16 H2 (LogEC50 = 1.32 ± 0.04).
