Abstract
Patterns of invasion and stromal response are understudied in vulvar squamous cell carcinoma (vSCC). The aim of this study was to explore whether histologic features such as an infiltrative pattern of invasion and fibromyxoid stromal response (FMX-SR) are meaningful prognostic factors. We reviewed 143 vSCC resections and correlated patterns of invasion and stromal response with patient age, ethnicity, depth of invasion (DOI), tumor size, perineural invasion (PNI) (S100/AE1/3 stain), lymph node involvement (LNI), extranodal extension, margin status, pathologic stage, and recurrence. Univariate analyses of continuous variables were performed using t-tests, while Pearson’s χ2 tests were used for categorical variables. Logistic regression analyses examined the relationship between histopathologic characteristics and clinical outcomes. There was a statistically significant association between infiltrative tumors and a FMX-SR in comparison to non-infiltrative tumors (p < 0.001). Tumors with FMX-SR were significantly more deeply invasive (p=0.0025) and more likely to have LNI (p=0.0364), extranodal extension (p=0.0227) and PNI (p=0.0011) compared to tumors without FMX-SR. For cases with negative surgical margins, the association between tumors with FMX-SR and LNI was significantly strengthened (OR=4.73, p=0.0042), even after adjustments for age, race and DOI (OR=4.34, p=0.0154). Presence of both FMX-SR and infiltrative pattern of invasion in tumors with negative margins was significantly associated with LNI (p=0.0235) and recurrence (p=0.0124). These results suggest that interactions between nerve, tumor, and stromal cells play a role in tumor progression and represent additional prognostic factors that help stratify those patients at highest risk for LNI, extranodal extension and recurrence.
Introduction
Vulvar cancer represents 0.3% of all new cancer diagnoses in the U.S. and an estimated 4,850 new cases were reported in 2014.1 It is primarily a disease of elderly women with those between the ages of 75 and 84 most commonly affected. The majority of vulvar cancers (90%) are squamous cell carcinomas.2 Although a relatively uncommon malignancy, the treatment for vulvar squamous cell carcinoma (vSCC) can be extremely disfiguring. Moreover, tumor recurrence can be a complicating factor, particularly in patients with tumors > 2.5 cm, multifocal disease, and involvement of surgical margins, lymph-vascular space invasion, or precursor lesions (high grade squamous intraepithelial lesions, VIN 2/3).2 While current clinical criteria for use of adjuvant post-surgical therapy for vSCC patients encompass presence or absence of nodal involvement, depth of invasion, and margin status, recurrence rates can reach as high as 50–70%.3,4 Therefore, a better risk stratification of patients with vSCC is needed.
Studies investigating prognostic factors in patients with vulvar cancer have led to the current staging system, which is based on tumor size (as measured clinically), depth of invasion, extent of regional involvement and lymph node status.5 Recent studies in other gynecologic malignancies such as endocervical adenocarcinoma and endometrial carcinoma have focused their attention on patterns of invasion and stromal response in order to gain a better understanding of tumor behavior in relation to outcome; however, few studies have addressed these histologic features in the arena of vulvar pathology.6–10
Although an infiltrative pattern of invasion in vSCC has been frequently cited as an adverse prognostic factor,11–15 the literature is particularly sparse in regards to the importance of stromal responses to vSCC. In a review of 51 cases of vSCC, Ambros and colleagues reported that a subset of vSCCs showed a prominent fibromyxoid stromal response, and that these tumors were more likely to be associated with women of older age, clitoral involvement, nodal metastasis and overall poorer survival rates compared to tumors without a prominent fibromyxoid stromal response.16 In a larger study of 184 cases of vSCC in Brazilian women, Pinto et al. found that a fibromyxoid stromal response was significantly associated with decreased survival using a univariate analysis, but was not significant using a multivariable Cox proportional hazard analysis.11
Given the sparse literature on patterns of tumor invasion and stromal response in vSCC, the aim of the current study was to examine the association between these histopathological features, and to assess their prognostic importance in a large series of consecutive resection specimens. Specifically, our study explores whether histologic features such as an infiltrative pattern of invasion and a fibromyxoid stromal response are meaningful prognostic factors that would potentially allow for better patient risk stratification and more effective treatment planning.
Materials and Methods
Clinicopathologic Parameters
One-hundred and forty-three cases of invasive squamous cell carcinoma of the vulva were retrieved from our archives. These represented consecutive resection specimens from 128 patients from 1997 through 2013. All available H&E stained slides from all cases were reviewed simultaneously by two gynecologic pathologists (CMQ and SKJ).
Electronic medical records and pathology reports were examined to record patient age, ethnicity, depth of invasion (DOI), tumor size, lymph node status, margin status, pathologic stage, recurrence, and clinical follow up.
Patterns of Invasion
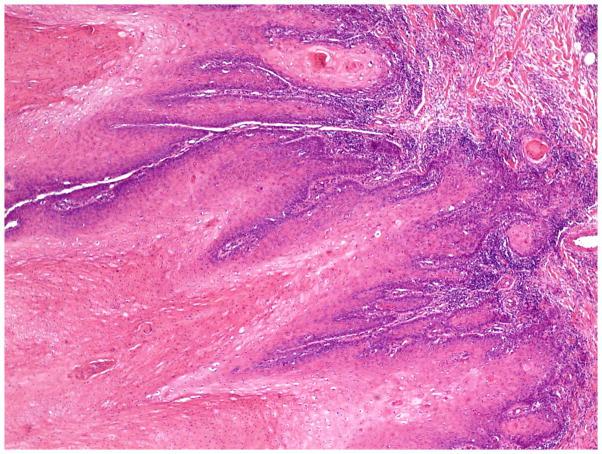
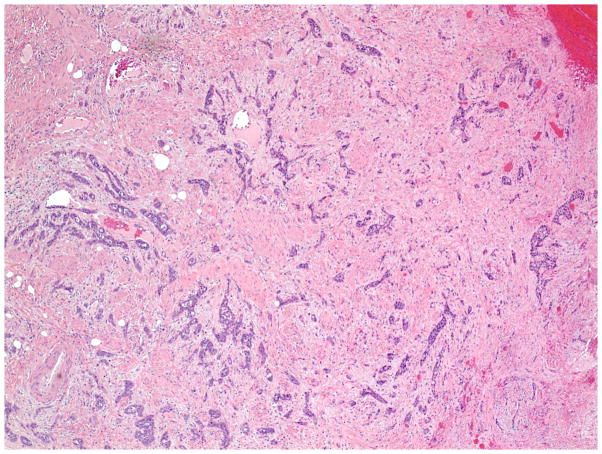
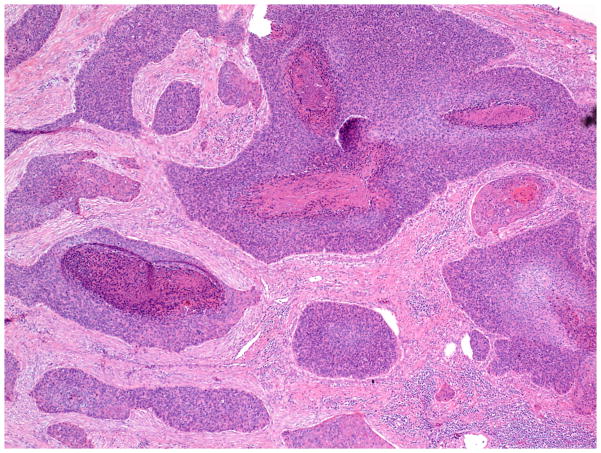
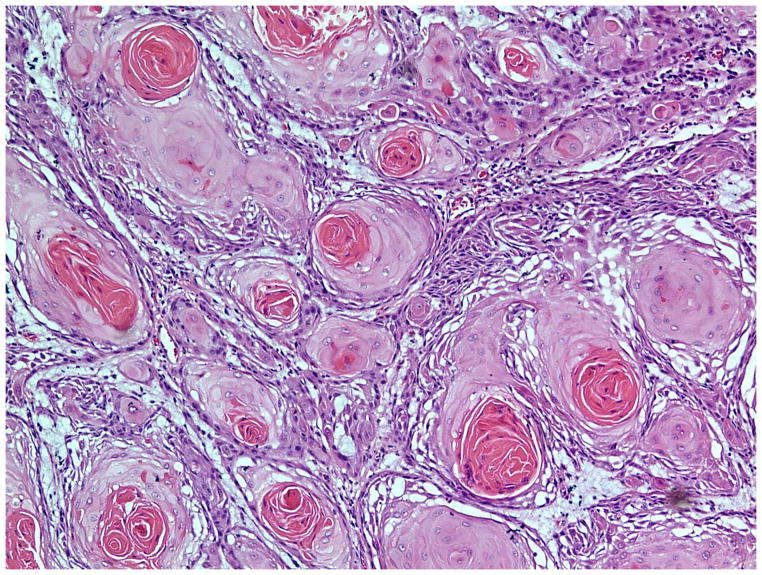
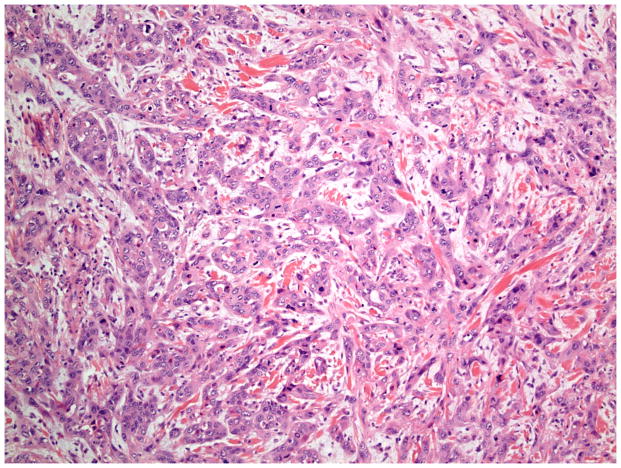
The invasive pattern of the tumors was classified as solid/pushing, nested, or infiltrative (Figure 1–4). Solid/pushing invasion consisted of tumor growing in a solid sheet, pushing into the underlying stroma (Figure 1). Nested invasion consisted either of large geographic nests with frequent central comedo-type necrosis (Figure 2) or small nests with frequent central keratin pearls (Figure 3). The infiltrative pattern of invasion was defined by poorly differentiated tumor cells invading the underlying stroma in strands, cords, and single tumor cells creating a spray–like pattern (Figure 4). Because not all tumors exhibited a single pattern, a secondary pattern was recorded if present. Pattern evaluations were performed at low power (4–10X) magnification.
Figure 1.
Invasive pattern: Solid/pushing. The tumor shows a solid growth pattern and invades the underlying stroma in a pushing manner. (H&E, 40x magnification)
Figure 4.
Invasive pattern: Infiltrative. The tumor cells are poorly differentiated and form strands and cords infiltrating the stroma in a “spray” pattern. (H&E, 20x magnification)
Figure 2.
Invasive pattern: Nested. The tumor invades the stroma as large geographic nests, many with central comedo-type necrosis. (H&E, 40x magnification)
Figure 3.
Invasive pattern: Nested. The tumor invades the stroma as small nests, many with central keratin pearls. (H&E, 40x magnification)
Patterns of Stromal Response
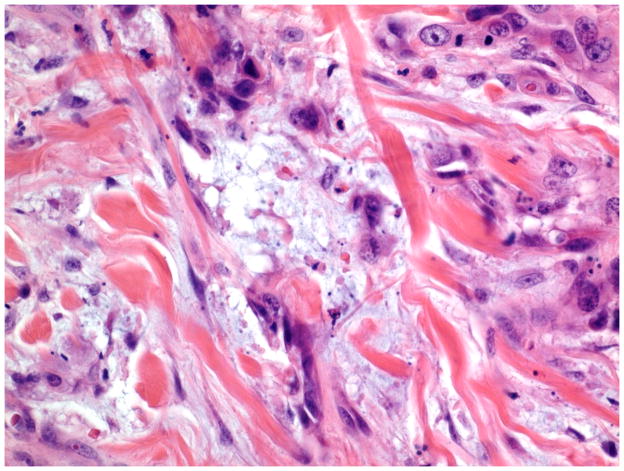
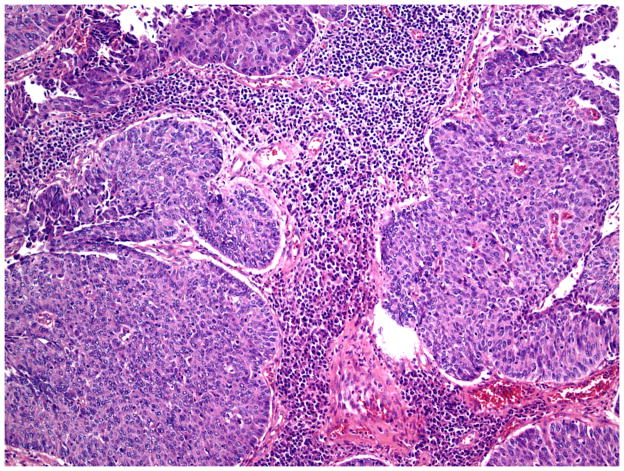
Stromal responses were recorded as present or absent and categorized as: fibromyxoid and/or inflammatory. As previously described, a fibromyxoid stromal response was defined as extracellular matrix composed of immature collagen and fibroblasts separating and surrounding the tumor cells (Figure 5).16 On high power examination, the myxoid quality of the stroma had a blue hue compared to the background stroma (Figure 6). Inflammatory stroma was defined as a prominent band-like infiltrate surrounding the tumor, which on high power was composed of lymphocytes and plasma cells (Figure 7). Stromal response assessments were performed at low power (4–10X magnification) and confirmed with high power examination (20X magnification).
Figure 5.
Stromal response: Fibromyxoid. (low power view). The stroma has a myxoid type quality imparting a bluish hue and consists of prominent extracellular matrix composed of immature collagen and fibroblasts separating the tumor cells. (H&E, 40x magnification)
Figure 6.
Stromal response: Fibromyxoid (high power view). (H&E, 400x magnification)
Figure 7.
Stromal response: Inflammatory. The stroma is composed of lymphocytes and plasma cells. (H&E, 400x magnification)
Lymph Node Involvement
All H&E stained slides from associated lymph node dissections were reviewed. For cases with metastatic involvement, the presence or absence of extranodal extension was documented and correlated with DOI, pattern of invasion and stromal response of the corresponding primary tumor.
Perineural Invasion
Presence or absence of perineural invasion (PNI) was determined for a subset of our dataset (n = 103) as previously described (Holthoff E, Jeffus S, Gehlot A, et al. Perineural invasion is an independent pathologic indicator of recurrence in vulvar squamous cell carcinoma. AJSP. In press, 2015). Briefly, dual-stain immunohistochemistry was performed with antibodies against cytokeratin AE1/3 (Dako M3515) and S100 (Dako Z0311) to identify epithelial and nerve cells, respectively. Each slide was reviewed for presence of PNI as previously defined17, and PNI was correlated with pattern of invasion and stromal response.
Statistical Analysis
Univariate analyses of continuous variables were performed using t-tests, and Pearson’s χ2 tests were used for categorical variables. Logistic regression analyses were used to examine the relationship between histopathologic characteristics (patterns of invasion and stromal response) and the dichotomous clinical outcomes of lymph node metastasis and disease recurrence. P-values <0.05 were considered to be indicative of statistically significant results.
Results
General Clinicopathologic Parameters
Patients ranged in age from 21 to 97 years old (mean: 63 years). Of the 128 patients studied, 116 (91%) were Caucasian, 11 were African American and 1 was unknown. Tumor size ranged from 0.3 – 11.0 cm (mean: 3.3 cm), with a DOI that ranged from < 1 mm to 64 mm (mean: 9.6 mm). Margin status was available for 142 cases, 30 of which demonstrated involvement of a surgical margin by tumor. Lymph nodes were sampled in 94 cases, 31 (33%) of which showed squamous cell carcinoma involvement in at least one lymph node. Tumor recurrence was noted in 42 (33%) of the 128 identified patients. Clinical stage was available for 137 cases and distributed as follows: IA-6, IB-73, II-23, III-25, and IV-10. Clinical surveillance data was available for 52% of our patients with a mean follow up of 28 months and a maximum of 132 months. Based on this surveillance data, 21 patients died of disease, 1 died of other cause, 3 are alive with disease, and 41 are alive without disease.
Patterns of Invasion
As described in methods, patterns of tumor invasion were categorized as solid/pushing (Figure 1), nested [large geographic nests (Figure 2) or small nests (Figure 3)] or infiltrative (Figure 4). Sixty two (43%) tumors contained an infiltrative pattern of invasion, of which 11 cases were purely infiltrative with the remainder displaying a mixed pattern (infiltrative component in combination with the solid/pushing or nested pattern). Tumors with any infiltrative component were labeled “infiltrative”. Eighty one (57%) cases lacked an infiltrative pattern and were purely solid/pushing, nested, or a combination of both. These cases were labeled as “non-infiltrative”.
The possible association of tumor invasive pattern with stromal response was then analyzed. We found that infiltrative tumors were significantly more often associated with the presence of a fibromyxoid stromal response than non-infiltrative tumors [54/62 (87%) vs 13/81 (16%), p < 0.001]. In contrast, 70% of the non-infiltrative tumors were accompanied by a prominent inflammatory stromal response compared with only 39% of infiltrative tumors [57/81 (74%) vs 24/62 (39%), p < 0.001].
To assess the prognostic importance of tumor patterns of invasion, we examined the association of the different tumor patterns with clinical outcomes. We found that infiltrative tumors had a significantly greater DOI compared to non-infiltrative tumors (12.6 mm vs 7.3 mm, p = 0.001). Although there was no significant association of tumor pattern of invasion with tumor size or lymph node status, tumors with the presence of any infiltrative component were 2-times more likely to be associated with a recurrence (OR=2.15, p=0.0459) than those without an infiltrative component (Table 1). This association remained strong when adjusted for age and race (OR=2.08, p=0.0571) in a multivariable logistic regression analysis. In cases with negative surgical margins the association between infiltrative pattern of invasion and tumor recurrence remained strong (OR=2.31, p=0.0441) (Table 2).
Table 1.
Evaluation of the association between specific histopathologic characteristics [fibromyxoid stroma (FMX) and infiltrative invasion] and outcomes of recurrence and lymph node metastasis. Adjusted odds ratio estimates were obtained using multivariable (MV) logistic regression models which, in addition to histopathology, included age, race and depth of invasion (DOI).
| Unadjusted Estimates | Adjusted Estimates | |||||
|---|---|---|---|---|---|---|
| Model A1 | Model B2 | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| LN Metastasis | ||||||
|
| ||||||
| FMX | 2.59 (1.06, 6.30) | 0.0364 | 2.43 (0.98, 6.05) | 0.1913 | 2.16 (0.85, 5.50) | 0.1049 |
| Infiltrative | 1.52 (0.64, 3.60) | 0.3441 | 1.38 (0.57, 3.37) | 0.4761 | 1.14 (0.45, 2.90) | 0.7809 |
| Both3 | 1.99 (0.83, 4.77) | 0.1235 | 1.83 (0.74, 4.57) | 0.1924 | 1.58 (0.62, 4.05) | 0.3418 |
| Either4 | 2.03 (0.83, 5.01) | 0.1222 | 1.89 (0.76, 4.73) | 0.1726 | 1.61 (0.63, 4.15) | 0.3199 |
|
| ||||||
| Recurrence | ||||||
|
| ||||||
| FMX | 2.25 (1.06, 4.77) | 0.0348 | 2.16 (1.01, 4.61) | 0.0470 | 1.95 (0.89, 4.24) | 0.0935 |
| Infiltrative | 2.15 (1.01, 4.55) | 0.0459 | 2.08 (0.94, 4.18) | 0.0571 | 1.86 (0.85, 4.06) | 0.1214 |
| Both3 | 2.68 (1.25, 5.76) | 0.0113 | 2.58 (1.19, 5.57) | 0.0159 | 2.33 (1.06, 5.15) | 0.0360 |
| Either4 | 1.87 (0.84, 3.70) | 0.1038 | 1.81 (0.85, 3.86) | 0.1265 | 1.60 (0.73, 3.51) | 0.2410 |
Estimates are adjusted for age and race.
Estimates are adjusted for age, race and DOI.
“Both” refers to samples having both FMX and Infiltrative characteristics versus those having only one or neither of the characteristics.
“Either” refers to samples having both or only one FMX or Infiltrative characteristics versus those having neither.
Table 2.
Association between specific histopathology characteristics and outcomes of recurrence and lymph node metastasis for patients with negative margins. Adjusted odds ratio estimates were obtained using multivariable (MV) logistic regression models which, in addition to histopathology, included age, race and depth of invasion (DOI).
| Unadjusted Estimates | Adjusted Estimates | |||||
|---|---|---|---|---|---|---|
| Model A1 | Model B2 | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| LN Metastasis (N=78) | ||||||
|
| ||||||
| FMX | 4.73 (1.52, 14.72) | 0.0042 | 4.66 (1.45, 14.98) | 0.0098 | 4.34 (1.32, 14.21) | 0.0154 |
| Infiltrative | 2.08 (0.82, 2.66) | 0.1560 | 1.96 (0.69, 5.61) | 0.2085 | 1.79 (0.61, 5.25) | 0.2878 |
| Both3 | 3.25 (1.15, 9.18) | 0.0235 | 3.21 (1.07, 9.66) | 0.0376 | 2.95 (0.96, 9.10) | 0.0597 |
| Either4 | 3.09 (1.01, 9.57) | 0.0399 | 2.96 (0.94, 9.28) | 0.0630 | 2.73 (0.86, 8.72) | 0.0895 |
|
| ||||||
| Recurrence (N=106) | ||||||
|
| ||||||
| FMX | 1.92 (0.86, 4.31) | 0.1133 | 1.72 (0.76, 3.94) | 0.1960 | 1.59 (0.69, 3.68) | 0.2810 |
| Infiltrative | 2.31 (1.02, 5.22) | 0.0441 | 2.08 (0.91, 4.78) | 0.0840 | 1.93 (0.83, 4.49) | 0.1292 |
| Both3 | 2.88 (1.26, 6.61) | 0.0124 | 2.54 (1.08, 5.97) | 0.0322 | 2.34 (0.98, 5.59) | 0.0550 |
| Either4 | 1.60 (0.71, 3.59) | 0.2546 | 1.47 (0.65, 3.36) | 0.3564 | 3.15 (0.59, 3.15) | 0.4711 |
Estimates are adjusted for age and race.
Estimates are adjusted for age, race and DOI.
“Both” refers to samples having both FMX and Infiltrative characteristics versus those having only one or neither of the characteristics.
“Either” refers to samples having both or only one FMX or Infiltrative characteristics versus those having neither.
Patterns of Stromal Response
Stromal responses to the tumor were categorized as fibromyxoid or inflammatory (Figures 5–7). Sixty seven (47%) cases showed a prominent fibromyxoid stromal response, of which 40 were purely fibromyxoid and 27 also contained a prominent inflammatory stroma. When both stromal reactions were present in the same tumor, they were mutually exclusive for any given area (e.g. an inflammatory stromal response was never superimposed on a fibromyxoid stromal response and vice versa). Seventy six (53%) cases exhibited no fibromyxoid stromal response, 54 of which showed a prominent inflammatory stroma and 22 had no stromal response.
To assess the prognostic importance of stromal responses, we examined the association of the different stromal responses with clinical outcomes. We found no significant differences that distinguished cases with an inflammatory stroma from cases with no stromal response (neither fibromyxoid nor inflammatory) with respect to any of our recorded clinicopathologic parameters. Therefore, we classified stromal responses as either “fibromyxoid stroma present” or “fibromyxoid stroma absent”. Using this classification, there was no significant association of stromal response and tumor size. However, we found that tumors with a fibromyxoid stroma, irrespective of the pattern of tumor invasion, were significantly more deeply invasive (12.2 mm vs 7.3 mm, p=0.0025); more likely to have lymph node involvement [20/46 (43%) vs 11/48 (23%), p=0.0364], and to recur [25/59 (42%) vs 17/69 (25%), p=0.0348] compared to tumors without a fibromyxoid stromal response (Table 1). Furthermore, a multivariable logistic regression model showed that tumors with both a fibromyxoid stroma and an infiltrative tumor morphology were 2.7-times (p = 0.0113) more likely to be associated with a recurrence than tumors with either one or neither of those features. This association was observed even after accounting for age, race and depth of invasion. (OR= 2.33, p = 0.0360) (Table 1).
When focusing the analysis solely on cases with negative surgical margins, the association between tumors with a fibromyxoid stromal response and lymph node involvement was significantly strengthened (OR=4.73, p=0.0042), even after adjustments for age, race and depth of invasion (OR=4.34, p=0.0154) (Table 2). However, the significance of association with recurrence was reduced (p=0.1133) (Table 2). Importantly, presence of both a fibromyxoid stroma and an infiltrative pattern of invasion in tumors with negative margins was significantly associated with both lymph node involvement (OR=3.25; p=0.0235) and recurrence (OR=2.88; p=0.0124), and remained significant after adjusting for age and race (Table 2).
Lymph Node Involvement and Extranodal Extension
In addition to assessing lymph node involvement, we examined whether the involved nodes had extranodal extension, which is an important indicator for use of adjuvant therapy. Thirty-one out of 94 (33%) lymph node dissections showed metastatic disease in at least one lymph node. One case represented a fine needle aspirate and was excluded from the assessment of extranodal extension. Of the remaining 30 lymph node dissections with metastatic disease, the average positive lymph node count was 2.46 (range 1–15) to a total lymph node count of 11.1 (range 1–26). Extranodal extension was present in 17/30 cases (57%). When correlated with DOI, pattern of invasion, or stromal response of the primary tumor, the presence of a fibromyxoid stroma was the only feature of the primary tumor that was significantly associated with extranodal extension in the metastasis (p=0.0227, Table 3).
Table 3.
Characteristics of primary tumor in relation to metastases with and without ECE.
| ECE (n) | DOI (mm, mean) | FMX stroma | Infiltrative growth |
|---|---|---|---|
| Yes (17) | 1.41 | 14 | 12 |
| No (13) | 1.25 | 5 | 5 |
| P value | 0.7407 | 0.0227 | 0.1376 |
Perineural Invasion
We have recently associated PNI with tumor recurrence in vSCC (Holthoff E, Jeffus S, Gehlot A, et al. Perineural invasion is an independent pathologic indicator of recurrence in vulvar squamous cell carcinoma. AJSP. In press, 2015), therefore we assessed whether PNI was associated with tumor pattern and stromal response. Tumors with an infiltrative pattern of invasion were significantly associated with PNI compared with non-infiltrative tumors [32/46 (70%) vs. 22/57 (39%), p=0.0028]. In addition, tumors with a fibromyxoid stromal response were more likely to contain PNI than tumors without a fibromyxoid response [28/38 (74%) vs 21/56 (38%), p=0.0029]; and tumors with both fibromyxoid stroma and an infiltrative pattern of invasion were highly associated with PNI when compared to tumors with one of those features or neither (p=0.0011).
Discussion
Histologic patterns of invasion and their associated stromal responses have recently emerged as a topic of discussion in the literature on gynecologic malignancies such as endocervical and endometrial carcinoma but are relatively understudied in the vulva.6–10 In 1987, Ross and Ehrmann studied 64 cases of stage I vSCC.18 Utilizing the descriptions by Barnes et al 19, vSCCs were divided according to three patterns of invasion: carcinoma in situ with early stromal invasion, pushing, and infiltrative. The pushing pattern was defined by a broad, smooth front of invasion and dissociated nests; the infiltrative pattern by ribbons, cords, or single cells. In their study, patients with carcinoma in situ with early stromal invasion had low risk for lymph node involvement and no increased recurrence risk. In addition, the pushing pattern of invasion was a favorable histologic feature, whereas the infiltrative pattern of invasion was predictive of lymph node involvement. Additional research has shown that the infiltrative pattern of invasion is associated with a higher likelihood of lymph node involvement and decreased overall survival; however, stromal responses to vSCC have been relatively understudied.12–15
In a review of 51 vSCC cases Ambros and colleagues observed that a subset of vSCC showed a prominent fibromyxoid stromal response, which they defined as a well-demarcated stromal reaction that consisted of a prominent extracellular matrix composed of immature collagen and fibroblasts separating the tumor cells.16 The authors semiquantitatively categorized the extent of this stromal response as focal (<25%), regional (26–50%), or diffuse (>50%). The fibromyxoid stromal response was identified in over half of cases with a focal, regional and diffuse distribution of 21%, 14%, and 16%, respectively. The remaining cases showed no fibromyxoid stromal response. There was no statistically significant difference when the fibromyxoid stromal response was categorized as “present” or “absent” and correlated with other clinicopathologic features including age, tumor location, gross appearance (exophytic or ulcerative), tumor grade, tumor size, depth of invasion (DOI), pattern of invasion (pushing, raggedly infiltrative, or mixed), type of vSCC (typical, basaloid, warty), lymph node status, recurrence, and overall survival. However, there were significant correlations when tumors with a focal fibromyxoid stromal response (<25%) were excluded from the analysis. Specifically, tumors with a prominent fibromyxoid stromal response (>25%) were more likely to be associated with older age, clitoral involvement, nodal metastasis and overall poorer survival rates compared to tumors without a prominent fibromyxoid stromal response. The authors concluded that only tumors with a prominent fibromyxoid stromal reaction might represent an aggressive subset of vSCC.
Pinto and colleagues studied the clinicopathologic features of vSCC in Brazilian women in a study of 184 cases.11 The authors examined the potential correlations between age, tumor grade, thickness, histologic pattern (warty/basaloid or keratinizing), pattern of invasion (blunt/nested, infiltrative, or mixed), stromal response (fibromyxoid or inflammatory), HPV status, pathologic stage, and overall survival. Increasing age, stage, grade, tumor thickness, and basaloid histology were each independently correlated with decreased survival. In addition, there was an association of the infiltrative pattern of invasion with increased mortality. Although a fibromyxoid stromal response was associated with a worse outcome in a univariate analysis, this association was not significant in a multivariable Cox proportional hazard analysis that adjusted for age, stage, tumor thickness, histologic type, pattern of invasion, number of keratin pearls, and HPV status. Importantly, Pinto et al. did not investigate the association of either invasive pattern or stromal response with tumor recurrence or nodal involvement.
Given this context, we reviewed 143 consecutive resection specimens of vSCC and explored the prognostic significance of patterns of invasion (infiltrative vs non-infiltrative) and stromal response (fibromyxoid stroma present vs absent). Our study is the first to report a strong association between tumors with an infiltrative pattern and a fibromyxoid stromal response. In addition, our results show that the presence of any infiltrative component in vSCC is significantly associated with more deeply invasive tumors and increased risk for recurrence compared to non-infiltrative tumors. Tumors lacking an infiltrative component (solid/pushing or nested tumors) were much more likely to demonstrate a prominent inflammatory infiltrate and a shallower depth of invasion. Moreover, in contrast to the findings of Ambros et al,16 our results show that the presence of any fibromyxoid stroma is an adverse prognostic factor, irrespective of the pattern of invasion. Specifically, tumors with a fibromyxoid stromal response were more deeply invasive and more likely to involve lymph nodes. The latter remained significant even after adjustment for age, race, depth of invasion and margin status in a multivariable analysis. Further, when nodal metastasis was present, tumors with a fibromyxoid stroma were more likely to demonstrate extracapsular extension. This is highly relevant because the presence of extracapsular extension is a reportable prognostic factor that is associated with poor outcome and independent indicator for increased dosage of adjuvant radiation. Although we report a strong association between an infiltrative pattern of invasion and a fibromyxoid stromal response, a small number (8) of pushing tumors also contained a fibromyxoid stromal response. Of these tumors, 50% (4 of 8) had lymph node involvement and were more likely to be associated with extracapsular extension, suggesting that stromal interactions may play a large role in the mechanism of tumor invasiveness.
In addition to pattern of invasion and stromal response, we recently identified that perineural invasion represents an independent risk factor for recurrence in vSCC (Holthoff E, Jeffus S, Gehlot A, et al. Perineural invasion is an independent pathologic indicator of recurrence in vulvar squamous cell carcinoma. AJSP. In press, 2015). Utilizing a dual immunostain (S-100/AE1/3), we showed that both the infiltrative pattern of invasion and a fibromyxoid stroma are strongly associated with perineural invasion. The association of PNI, infiltrative pattern of invasion and fibromyxoid stromal response in vSCC suggests that interactions between nerve, tumor, and stromal cells play a role in progression and recurrence of this cancer. The interplay among these cells will be a focus of future studies.
The ability of a tumor to invade and metastasize is partly due to its relationship with the surrounding stroma. Tumor-stromal interactions in vSCC are currently poorly understood. The specific cellular interactions that trigger a well-differentiated solid/pushing or nested tumor to become poorly differentiated and infiltrative remain to be explored. In addition, why infiltrative tumors show such a high association with the fibromyxoid stromal response is also a question requiring more inquiry. Therefore, more research is needed to define the tumor-stroma interactions in vSCC at the cellular level.
A major strength of our study is its large sample size (143 cases) of consecutive resection specimens. In addition, we purposefully chose not to semiquantitatively assess patterns of invasion or stromal response as was done in previous studies. In surgical pathology, it is most practical when a specific parameter is assessed as “present” or “absent”. Hence, we chose to analyze our data in this regard: infiltrative pattern present or absent as well as fibromyxoid stroma present or absent. Our results indicate that low power recognition of any infiltrative pattern and any fibromyxoid stromal response is important. This contrasts to previous studies by Ambros et al and Pinto et al in which the fibromyxoid stromal response was assessed in a semiquantitative fashion and only reached statistical significance when it was regional or diffuse (> 25%). This should prompt the surgical pathologist to focus not only on tumor morphology but also quality of the stroma.
Due to its retrospective design, our results are limited by the lack of clinical follow-up and information related to HPV status, tumor grade, and precursor lesions. The importance of HPV status and tumor grade as prognostic factors in vSCC remains controversial because conflicting results have been reported.11, 20, 21 Regarding tumor grade, a uniform grading system for vulvar squamous cell carcinoma has not been unanimously accepted, and grading has not been shown to demonstrate prognostic significance.22 Precursor lesions correlate with histologic type of vSCC and a significant correlation between lichen sclerosus and subsequent vSCC with a fibromyxoid stromal response has been reported.23 More studies are needed to address the interactions between HPV status, precursor lesions, pattern of invasion and stromal response in relation to tumor behavior, and we hope to address several of these issues in subsequent projects.
In summary, we are the first to report that a fibromyxoid stromal response is highly associated with an infiltrative pattern of invasion and perineural invasion in vSCC. In addition, the results of our study indicate that tumors with an infiltrative pattern of invasion, a fibromyxoid stromal response, and perineural invasion represent an important subset of vulvar squamous cell carcinomas that behave more aggressively. In particular, an infiltrative pattern of invasion and perineural invasion are highly associated with an increased risk of tumor recurrence, whereas a fibromyxoid stromal response is associated with increased risk of nodal metastases. These additional prognostic factors may help stratify those patients at highest risk for lymph node involvement, extranodal extension and recurrence. A better stratification of risk will be valuable in surgical planning and in increasing the effective use of adjuvant therapy.
Acknowledgments
This study was supported by the Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences and an institutional training grant T32GM106999.
Footnotes
Conflict of Interest:
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Surveillance, Epidemiology, and End Results Program. National Cancer Institute; 2014. [accessed on 12/20/2014]. SEER Cancer Statistics Factsheets: Vulvar Cancer. Available at: http://seer.cancer.gov/statfacts/html/vulva.html. [Google Scholar]
- 2.Eifel PJ, Berek JS, Markman MA. Cancer of the cervix, vagina, and vulva. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 1311–44. [Google Scholar]
- 3.Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000;89:116–22. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Piura B, Masotina A, Murdoch J, et al. Recurrent squamous cell carcinoma of the vulva: a study of 73 cases. Gynecologic oncology. 1993;48:189–95. doi: 10.1006/gyno.1993.1032. [DOI] [PubMed] [Google Scholar]
- 5.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 6.Diaz De Vivar A, Roma AA, Park KJ, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. 2013;32:592–601. doi: 10.1097/PGP.0b013e31829952c6. [DOI] [PubMed] [Google Scholar]
- 7.Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003;22:324–33. doi: 10.1097/01.pgp.0000092161.33490.a9. [DOI] [PubMed] [Google Scholar]
- 8.Hertel JD, Huettner PC, Pfeifer JD. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int J Gynecol Pathol. 2014;33:127–34. doi: 10.1097/PGP.0b013e318285657b. [DOI] [PubMed] [Google Scholar]
- 9.Quick CM, May T, Horowitz NS, et al. Low-grade, low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31:337–43. doi: 10.1097/PGP.0b013e31823ff422. [DOI] [PubMed] [Google Scholar]
- 10.Zaino RJ. Unusual patterns of endometrial carcinoma including MELF and its relation to epithelial mesenchymal transition. Int J Gynecol Pathol. 2014;33:357–64. doi: 10.1097/PGP.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 11.Pinto AP, Signorello LB, Crum CP, et al. Squamous cell carcinoma of the vulva in Brazil: prognostic importance of host and viral variables. Gynecologic oncology. 1999;74:61–7. doi: 10.1006/gyno.1999.5458. [DOI] [PubMed] [Google Scholar]
- 12.Drew PA, al-Abbadi MA, Orlando CA, et al. Prognostic factors in carcinoma of the vulva: a clinicopathologic and DNA flow cytometric study. Int J Gynecol Pathol. 1996;15:235–41. doi: 10.1097/00004347-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Husseinzadeh N, Zaino R, Nahhas WA, et al. The significance of histologic findings in predicting nodal metastases in invasive squamous cell carcinoma of the vulva. Gynecologic oncology. 1983;16:105–11. doi: 10.1016/0090-8258(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 14.Kurzl R, Messerer D. Prognostic factors in squamous cell carcinoma of the vulva: a multivariate analysis. Gynecologic oncology. 1989;32:143–50. doi: 10.1016/s0090-8258(89)80025-3. [DOI] [PubMed] [Google Scholar]
- 15.Yoder BJ, Rufforny I, Massoll NA, et al. Stage IA vulvar squamous cell carcinoma: an analysis of tumor invasive characteristics and risk. The American Journal of Surgical Pathology. 2008;32:765–72. doi: 10.1097/PAS.0b013e318159a2cb. [DOI] [PubMed] [Google Scholar]
- 16.Ambros RA, Malfetano JH, Mihm MC., Jr Clinicopathologic features of vulvar squamous cell carcinomas exhibiting prominent fibromyxoid stromal response. Int J Gynecol Pathol. 1996;15:137–45. doi: 10.1097/00004347-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 18.Ross MJ, Ehrmann RL. Histologic prognosticators in stage I squamous cell carcinoma of the vulva. Obstetrics and gynecology. 1987;70:774–84. [PubMed] [Google Scholar]
- 19.Barnes AE, Crissman JD, Schellhas HF, et al. Microinvasive carcinoma of the vulva: a clinicopathologic evaluation. Obstetrics and gynecology. 1980;56:234–8. [PubMed] [Google Scholar]
- 20.Monk BJ, Burger RA, Lin F, et al. Prognostic significance of human papillomavirus DNA in vulvar carcinoma. Obstetrics and gynecology. 1995;85:709–15. doi: 10.1016/0029-7844(95)00045-s. [DOI] [PubMed] [Google Scholar]
- 21.Hording U, Kringsholm B, Andreasson B, et al. Human papillomavirus in vulvar squamous-cell carcinoma and in normal vulvar tissues: a search for a possible impact of HPV on vulvar cancer prognosis. Int J Cancer. 1993;55:394–6. doi: 10.1002/ijc.2910550310. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson E. Premalignant and Malignant Tumors of the Vulva. In: Kurman R, Ellenson L, Ronnett B, editors. Blaustein’s Pathology of the Female Genital Tract. New York: Springer; 2011. pp. 71–72. [Google Scholar]
- 23.Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Human pathology. 1998;29:932–48. doi: 10.1016/s0046-8177(98)90198-8. [DOI] [PubMed] [Google Scholar]