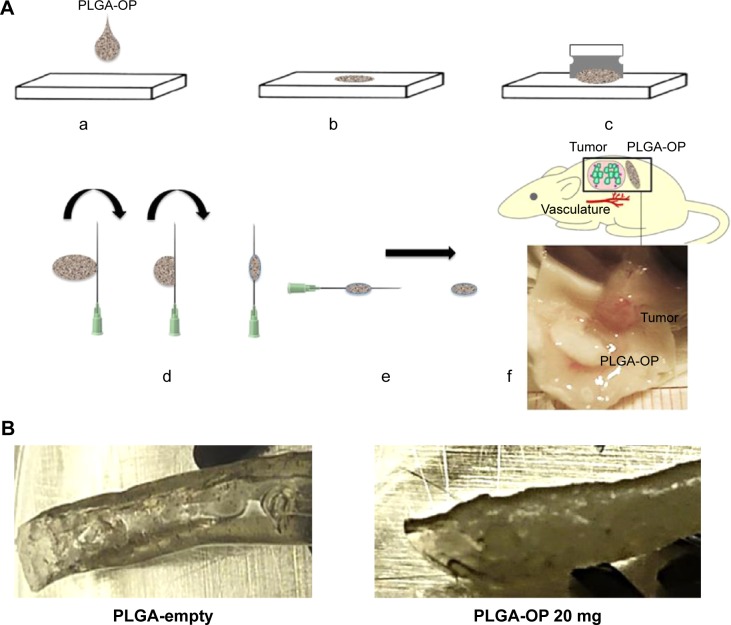
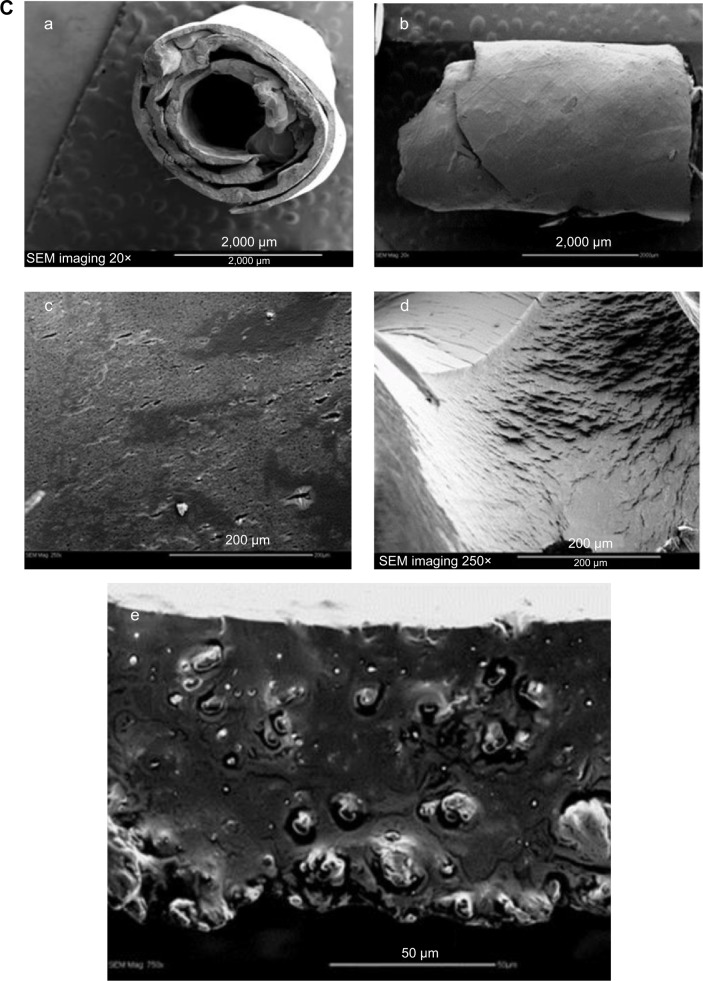
Figure 1.
Therapeutic design of PLGA-OP cylindrical implants.
Notes: (A) Fabrication of PLGA-OP; (a, b) dissolved suspension of PLGA in acetone containing Span 80 with OP or without as a blank was transferred using a 1 mL glass syringe from a height of 4 cm onto a smooth Teflon sheet to form a flat, circular disk, in a fume hood for 1 hour followed by refrigeration at 5°C overnight; (c) PLGA-OP disk was lifted from the teflon sheet using a razor blade, and (d) rolled onto a glycerol lubricated precision glide 18 gauge syringe tip to form a cylinder; (e) fabricated PLGA-OP cylinders were extracted from the syringe needle and stored at −80°C; (f) surgical implantation of blank PLGA and PLGA-OP at tumor site (image showing PLGA-OP near necropsied live tumor at end point of experiment. (B) Photograph of PLGA-empty and PLGA-OP cylinders. (C) SEM micrographs of PLGA-OP cylinder; (a) micrograph of a single layer cross-section with hollow continuous center throughout the entire PLGA-OP structure, (b) top surface, (c) magnified porous surface structure, (d) magnified surface structure, and (e) magnified internal structure of PLGA-OP showing crystals of OP.
Abbreviations: OP, oseltamivir phosphate; PLGA, poly (lactic-co-glycolic acid); SEM, scanning electron microscope.