Figure 2. Predicted structures of polycystin-1 (PC1), left, and polycystin-2 (PC2), right.
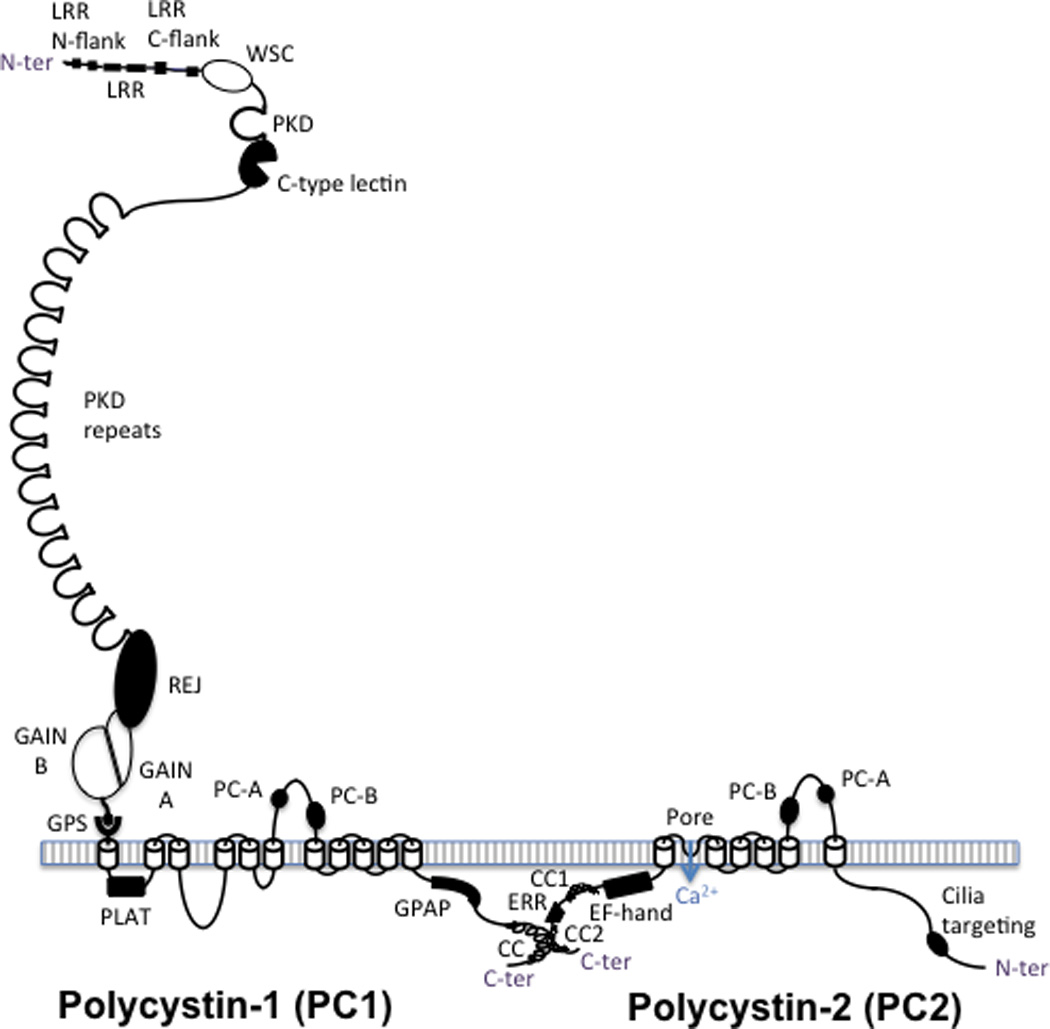
PC1 has a large ectodomain with a number of recognized domains sequentially located from the N terminus of the protein (after the signal peptide is cleaved): leucine rich repeats (LRR), flanked by N and C terminal domains, the cell wall integrity and stress-response component (WSC) domain, polycystic kidney disease (PKD) protein repeats, a C-type lectin, the receptor for egg jelly (REJ) domain, the G-protein coupled receptor (GPCR) proteolysis site (GPS), where PC1 is cleaved, and the associated GPCR autoproteolysis inducing (GAIN) domain, with A and B subdomains. PC1 is associated with the membrane with 11 transmembrane domains with the PC1, lipoxygenase, alpha-toxin (PLAT) domain found in the first cytoplasmic loop. The last 6 transmembrane region of PC1 is homologous to the transmembrane region of PC2, with the polycystin homologous motifs A and B found in the large extracellular loops of both proteins. The ∼200aa cytoplasmic tail contains a G-protein activating peptide (GPAP) and a coiled coil (CC) domain that interacts with the second coiled coil domain (CC2) found in the C-ter tail of PC2. PC2 is a TRP-like calcium channel that has an EF-hand and an endoplasmic reticulum retention (ERR) signal also in the C-tail and a proposed cilia targeting sequence in the N-terminal tail.
