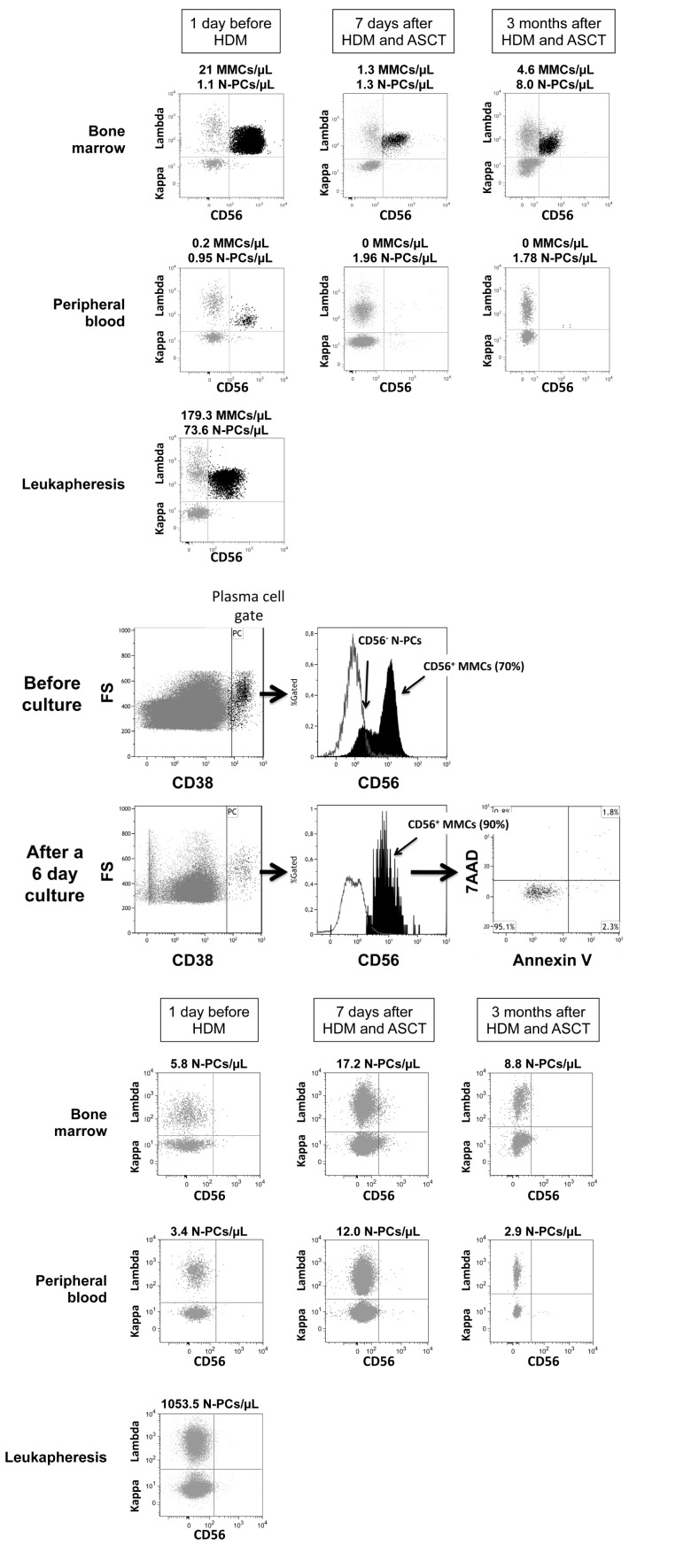
Figure 2. Assessment of Multiple Myeloma Cells and Normal Plasma cells in representative patients with Multiple Myeloma before and after high dose melphalan.
Using 7 color-multiparameter flow cytometry, multiple myeloma cells (MMCs) and normal plasma cells (N-PCs) were assessed in bone marrow or peripheral blood samples of patients after induction treatment (1 day before high dose melphalan, HDM), 7 days after HDM and autologous hematopoietic stem cell transplantation (ASCT), 3 months after HDM. MMCs and N-PCs were also measured in the thawed stem cell leukapheresis product grafted to the patients. (A) MMC and N-PC evaluation in a representative patient with positive residual disease after induction treatment (MRD+). MMCs were identified on the basis of aberrant CD56 expression and monoclonal lambda light chain expression. Data are the dotplots of CD56 and Lambda light chain expressions. MMCs were undetectable 7 days and 3 months after HDM+ASCT in the peripheral blood. (B) Bone marrow cells from one representative MRD+ patient out of 3 were harvested 7 days after HDM+ASCT and cultured for 6 days with 2 ng/mL of IL-6. Before culture, bone marrow cells contained CD38high CD56+ MMCs (70% of CD38high cells) and CD38highCD56− N-PCs. After a 6-day, the CD38high CD56+ MMCs were viable, being Annexin V− 7AAD−. (C) N-PC evaluation and lack of MMCs in a representative patient with negative residual disease after induction treatment. MMCs of this patient aberrantly expressed CD56 and CD200 at diagnosis. After induction treatment, only N-PCs could be detected in the bone marrow, peripheral blood and leukapheresis product.