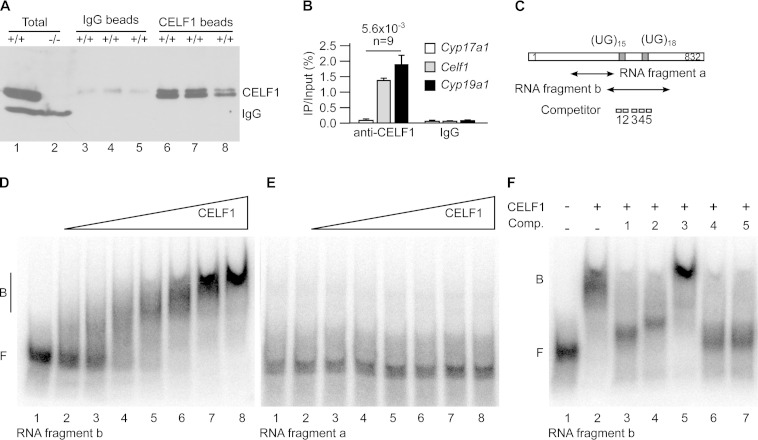
FIG 4.
CELF1 directly binds to Cyp19a1 mRNA. (A and B) We irradiated freshly dilacerated testes with UV light and carried out immunoprecipitations with either anti-CELF1 or nonimmune immunoglobulins (IgG, mock). (A) Western blots with anti-CELF1 antibodies of total testis extracts and of immunoprecipitates obtained with nonimmune and anti-CELF1 antibodies. The results of three representative independent immunoprecipitation experiments are shown. Except where indicated (−/−), all the testes were from wild-type mice. The positions of CELF1 and of the endogenous immunoglobulins detected by the secondary antibody are indicated. (B) Quantification by RT-qPCR of the coimmunoprecipitated mRNAs. The abundance of Cyp17a1 (negative control), Celf1 (positive control), and Cyp19a1 mRNAs in the immunoprecipitates relative to the inputs is shown. n, number of independent immunoprecipitation experiments. (C) Schematic drawing of the 3′ untranslated region of murine Cyp19a1 mRNA. The positions of the two UG stretches potentially able to interact with CELF1 and the positions of the fragments and the competitor oligonucleotides for electrophoretic mobility shift assays (EMSAs) are shown. (D and E) EMSAs using increasing amounts of recombinant CELF1 protein and the labeled RNA fragment b (D) or a (E). The fragment names are those used in panel C. (F) EMSA using recombinant CELF1 protein and the RNA fragment b, plus the indicated competitor oligonucleotides (Comp. 1 to 5).