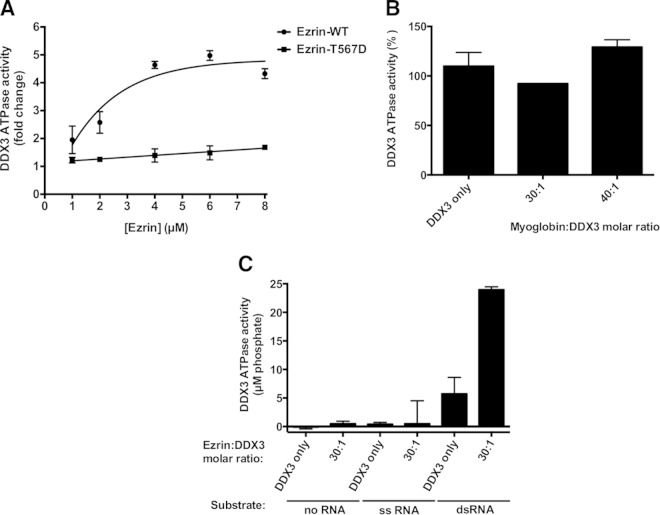
FIG 8.
Wild-type ezrin stimulates ATPase activity of DDX3 more effectively than the phosphomimicking ezrin T567D mutant in a concentration-dependent manner. (A) The effect of ezrin on DDX3 ATPase activity was determined using a fixed-type assay by measuring the amount of free phosphate generated during the hydrolysis of ATP, as described in Materials and Methods. Reaction mixtures containing 200 nM DDX3 and 400 nM dsRNA with a 3′ overhang were incubated with increasing amounts of either recombinant wild-type ezrin or the phosphomimicking ezrin T567D mutant (1 to 8 μM, corresponding to 5- to 40-fold molar excesses of ezrin over DDX3). The graphs are representative of the results of three independent experiments. (B) When myoglobin was added to the reaction mixtures as a negative-control protein at increasing concentrations corresponding to 30- and 40-fold molar excesses over the amount of DDX3, no apparent change in enzyme activity was observed, whereas ezrin at the same concentrations significantly stimulated DDX3 ATPase activity. The results are expressed as means and standard deviations of duplicate determinations. (C) Reaction mixtures containing 200 nM DDX3 were incubated with 6 μM recombinant wild-type ezrin (corresponding to a 30-fold molar excess of ezrin over DDX3) without any RNA substrate or in the presence of 400 nM either dsRNA with a 3′ overhang or its complementary, longer ssRNA. The sequence of the dsRNA is given in Materials and Methods.