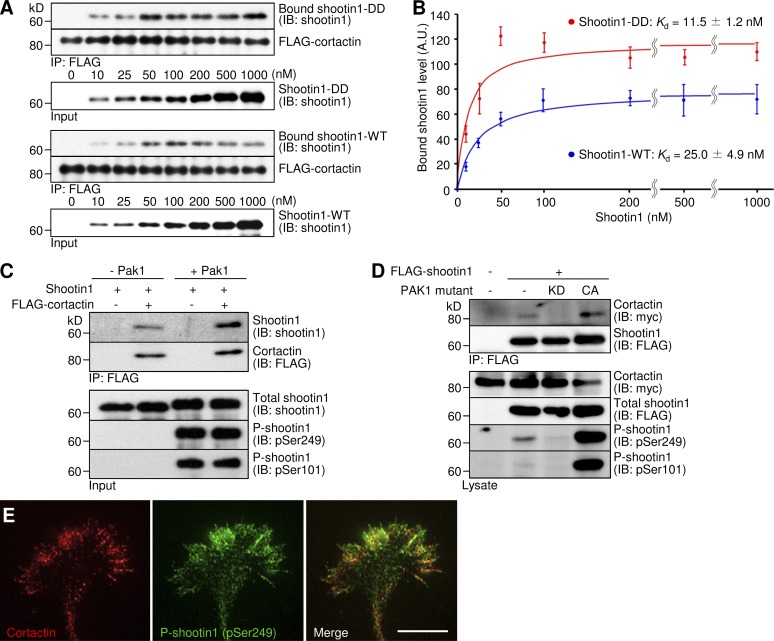
Figure 5.
Pak1-mediated shootin1 phosphorylation enhances shootin1–cortactin interaction. (A and B) In vitro binding assay using purified shootin1 and purified FLAG-cortactin. Shootin1-DD or shootin1-WT at increasing concentrations were incubated with FLAG-cortactin and anti-FLAG antibody. The immunoprecipitates were immunoblotted with anti-shootin1 or anti-FLAG antibody (A), and the bound shootin1-DD and -WT were then quantified (B). Data represent means ± SEM (error bars; n = 4). (C) In vitro binding assay using purified Pak1-phosphorylated shootin1 and purified FLAG-cortactin. Shootin1-WT (80 nM) or Pak1-phosphorylated shootin1-WT (80 nM) were incubated with FLAG-cortactin (80 nM) and anti-FLAG antibody. The immunoprecipitates were immunoblotted with anti-shootin1, anti-pSer249-shootin1, anti–pSer101-shootin1, or anti-FLAG antibody. (D) Coimmunoprecipitation of shootin1 and cortactin in COS7 cells. Cells were transfected with vectors to express FLAG-shootin1 and myc-cortactin; some of them were also cotransfected with a vector to express dominant-negative Pak1 (KD) or constitutively active Pak1 (CA) as indicated. Cell lysates were then incubated with anti-FLAG antibody. The immunoprecipitates were immunoblotted with anti-myc, anti-FLAG, anti-pSer249-shootin1, or anti–pSer101-shootin1 antibody. (E) Fluorescence images of an axonal growth cone costained with anti-cortactin (red) and anti–pSer249-shootin1 (green) antibodies. Bar, 5 µm.