Fig. 2.
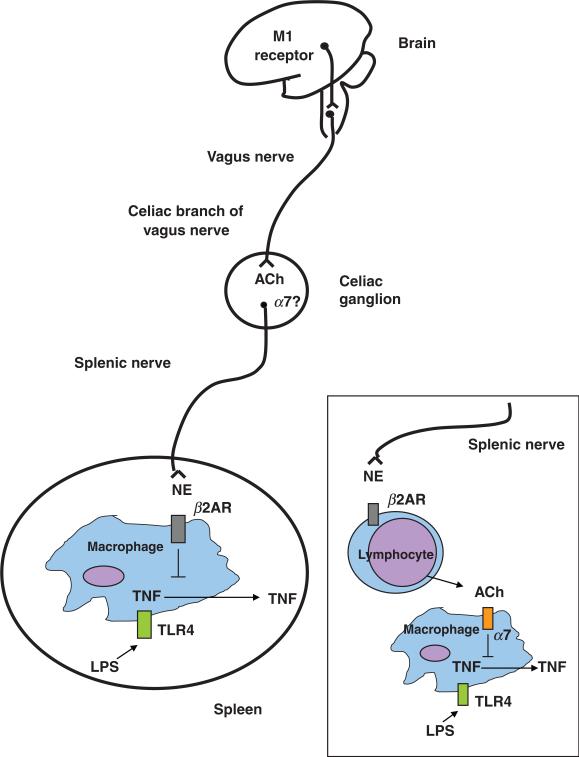
The cholinergic anti-inflammatory pathway, the efferent arm of the inflammatory reflex, is composed of the vagus nerve and its major neurotransmitter, acetylcholine. Electrical stimulation of the cervical vagus nerve attenuates systemic TNF through a pathway that requires the α7 subunit of the nicotinic acetylcholine receptor. Administration of α7 agonists or activation of a brain cholinergic network that depends on M1 muscarinic receptors and increases vagus nerve activity, attenuate systemic TNF levels. Two-neuron model of vagus nerve modulation of cytokine production via the splenic nerve: the preganglionic neuron, originates in the dorsal motor nucleus of the vagus; the postganglionic neuron, located in ganglia of the celiac-superior mesenteric plexus, reaches the spleen through the splenic nerve. In this model, electrical stimulation of the cervical vagus nerve attenuates systemic TNF through a pathway that requires the α7 subunit of the nicotinic acetylcholine receptor, the splenic nerve and catecholamines. Vagus nerve firing would modulate norepinephrine release by the splenic nerve. In this scenario, release of norepinephrine by the splenic nerve would act on β2-adrenergic receptors expressed on macrophages to attenuate TNF, and α7 expressed on neurons of the celiac/superior mesenteric plexus would convey signals between the vagus and the splenic nerve. An alternate possibility is that norepinephrine originating from splenic nerve terminals induces release of acetylcholine from cell sources other than neurons (e.g. lymphocytes), which would then act on α7 expressed on macrophages to attenuate TNF. NE, norepinephrine; β2AR, beta2-adrenergic receptor; ACh, acetylcholine.