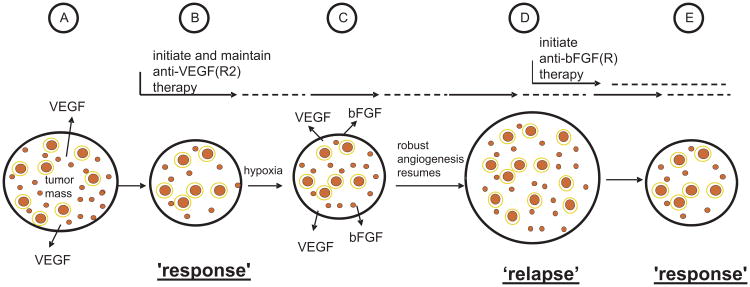
Fig. 2.
Schematic representation of one of the ways resistance can develop, in principle, to a targeted antiangiogenic drug in tumors which initially respond to the drug, e.g. anti-VEGF or anti-VEGFR-2 antibody, as exemplified by the results of Casanovas et al.31 Angiogenesis in untreated tumors (A) is driven mainly, for example, by VEGF; upon treatment with an agent such as an anti-VEGFR-2 antibody (B), some regression of newly formed immature tumor neovasculature (small red circles) occurs, and further angiogenesis is blunted along with reduced perfusion/flow in some remaining vessels, many of which are more mature, pericyte covered vessels (larger red circles with a yellow border to symbolize pericyte coverage) leading to a tumor ‘response’, e.g. a reduction in tumor mass or no new growth (“stable disease”); however, these effects on the tumor vasculature lead to an overall increase in the levels of tumor hypoxia which in turn leads to induction of expression of new hypoxia regulated proangiogenic growth factors, such as bFGF (C); the induction of bFGF induces angiogenesis despite ongoing anti-VEGFR-2 therapy, leading to tumor ‘relapse’, i.e., resumption of angiogenesis and robust expansion of tumor mass (D). Initiation of bFGF(R) directed antiangiogenic therapy at this point could lead to angiogenesis inhibition once again and a tumor response (E). Taken and adapted from Kerbel RS; Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed.