Table 2.
Effect of the Chiral Auxiliary on Selectivitya
| entry | enamides | products | time [h] | yield [%]b[dr]c |
|---|---|---|---|---|
| 1 |
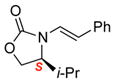 3b |
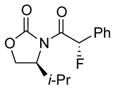 4b |
14 | 58 [93:7] |
| 2 |
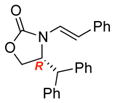 3c |
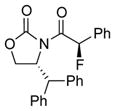 4c |
12 | 51 [91:9] |
| 3 |
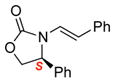 3d |
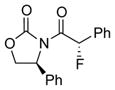 4d |
12 | 72 [>95:5] |
| 4 |
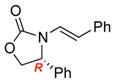 ent-3d |
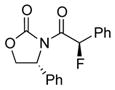 ent-4d |
12 | 63 [>95:5] |
Reaction condition: 1.1 equiv NFSI, 2 % H2O in CH3CN, 40 °C; and then, DMP, NaHCO3, CH2Cl2, rt.
Isolated yields.
Diastereomeric ratios [dr] were determined by 1H or/and 19F NMR spectroscopy.
