Figure 3. Increased Multiorgan Metastasis in Mice after Short-Term Sunitinib Treatment.
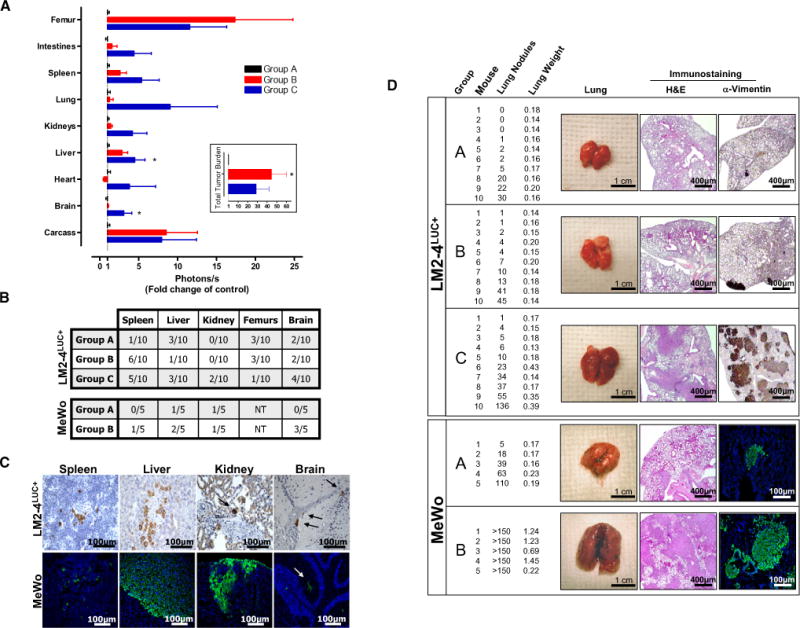
(A) Following the same experimental design as described in Figure 1A, SCID mice were sacrificed at day 27 to compare increases in overall tumor burden after short-term sunitinib treatment (inset) and corresponding increased bioluminescence in multiple organs (groups A, B, and C). Data are presented as mean ± SEM. Instances of highly divergent bioluminescence values did not permit statistical significance to be reached in all groups. 0.01 < *p < 0.05 by one-way ANOVA.
(B) Micrometastases were confirmed by immunostaining for human vimentin in organs of the 231/LM2-4LUC+ tumor model in (A) (upper panel) or in nu/nu mice receiving vehicle (group A) or short-term sunitinib therapy (group B) prior to intravenous (i.v.) inoculation with human MeWo melanoma cells (lower panel). Tissue sections were scored as positive or negative based on the presence or absence of detectable micrometastases. NT = not tested.
(C) Representative examples of micrometastases in spleen, liver, kidney, and brain shown using human-specific vimentin antibodies.
(D) Excised lungs from 231/LM2-4LUC+ and MeWo tumor models were scored visually for surface tumor nodules, with confirmation of macrometastasis by hematoxylin and eosin (H&E) and anti-vimentin immunostaining (representative images shown). For groups A, B, and C in the 231/LM2-4LUC+ tumor model, n = 10 per group. For groups A and B in the MeWo tumor model, n = 5 per group.
