Abstract
Metastasis is deadly and also tough to treat as it is much more complicated than the primary tumour. Anti-metastasis approaches available so far are far from being optimal. A variety of nanomedicine formulas provide a plethora of opportunities for developing new strategies and means for tackling metastasis. It should be noted that individualized anti-metastatic nanomedicines are different from common anti-cancer nanomedicines as they specifically target different populations of malignant cells. This review briefly introduces the features of the metastatic cascade, and proposes a series of nanomedicine-based anti-metastasis strategies aiming to block each metastatic step. Moreover, we also concisely introduce the advantages of several promising nanoparticle platforms and their potential for constructing state-of-the-art individualized anti-metastatic nanomedicines.
1. Introduction
Cancer is the deadliest disease worldwide accounting for 8.2 million deaths and 14.1 million new cancer cases in 2012. There will be over 20 million new cancer cases by 2025 according to the World Cancer Report 2014 from the World Health Organization (WHO). One of biggest barriers in cancer treatment is tumor metastsis, which is responsible for more than 90% of the death of cancer patients. Metastasis is an advanced progression of tumor, and the metastatic tumor is much more complicated than the primary tumor. Anti-metastasis, which is defined as the inhibition of any step of the metastasis cascade by metastasis diagnosis and therapy, is therefore much different from anti-primary tumor although both of which are involved in the treatment of cancer. Metastasis diagnosis and therapy is indeed vitally important but challenging because of the high complexity of the biological processes involved in metastasis. A small breakthrough in anti-metastasis might result in a major achievement in the clinical treatment of cancer. Only if effective broad-spectrum anti-metastasis drugs are discovered or individualized anti-metastasis treatments work, the survival rate of cancer patients may rise significantly.1
Current specific anti-metastasis treatments mainly bank on anti-vasculature (anti-angiogenesis) and matrix metalloproteinase (MMP) inhibitors. Most FDA-approved anti-metastasis drugs are classified into these two categories. However, these drugs are far from being satisfactory in the clinic because of the non-specific toxicities resulted from the lack of effective recognitions over the marker expression differences between metastases and normal tissues/cells. One solution to reduce drug toxicity is the employment of nanomedicine to optimize drug distribution, target tumour sites more efficiently, remotely deliver agents under imaging guidance and locally release drugs on demand, which involves the preparation of desired functional nano-carriers as well as the integration of nano-carriers with drugs, targeting molecules and other functional elements such as imaging agents for constructing individualized nanomedicines.
Nanotechnology is a vigorous technology defined as the manipulation of matter with at least one dimension sized from 1 nm to 100 nm. By applying advanced nanotechnologies, a wide range of new nanomaterials are being created. Emerging functional nanomaterials provide new platforms for biomedical applications, arousing a new wave of biological innovation. Especially in the field of cancer treatment and diagnosis, anti-cancer nanomedicines can be constructed by integrating nanomaterials as carries with drugs and/or imaging contrast agents. Nanomedicines exhibit several outstanding advantages over conventional chemotherapeutics: 1) the enhanced permeability and retention (EPR) effect in favour of passive tumour targeting; 2) the easy surface modification for active tumour targeting by conjugating targeting molecules and responsive drug release by coating sensitive molecules on the surface of nanoparticles; 3) the morphological and structural tunability at nanoscale for endocytosis and controlled drug release; 4) the facile integration of nano-carries with various drug molecules and imaging agents for nano-theranostics, etc. According to different requirements for individualized therapy, nano-carriers can be tailored to obtain expected physico-chemico-biological characteristics. For example, porous nano-structures have been created for controlled drug release,2–4 and hierarchical porous nano-structures have been engineered for co-delivery of different sized multi-drugs such as chemotherapeutics, genes and proteins;3–6 a hollow nano-structure enables remarkably enhanced payload of drug and co-delivery of hydrophobic and hydrophilic drugs;7–9 whereas a rattle-type porous nano-structure with a mesoporous shell and a functionalized fluorescent/magnetic/heavy core can realize simultaneous drug delivery and multimodality imaging;10–12 the intracellular and intranuclear uptake efficiencies of nanomedicines and their biodistributions can be controlled by tuning their particle sizes and surface properties. More features and advantages of several promising nanoparticle platforms for the construction of nanomedicines are summarized in Section 2.
Thanks to advanced nanotechnology, many types of nanomedicines against primary tumours have been developed, but the exploitation of anti-metastasis nanomedicines is still in its infancy. With the diversified development of anti-metastasis strategies and the emergence of various novel nanomaterials and nanotechnologies, we believe that the development of anti-metastasis nanomedicines and nanomedicine-based anti-metastasis strategies based on the metastatic cascade would receive more attention in future anti-cancer research and practice. This review aims to provide an overview of various nanomedicine-based anti-metastasis strategies, and facilitate experts in the fields of chemistry, cancer biology and nanomedicine to explore individualized anti-metastasis nanomedicines. We hope that our review could raise more interest for oncologists, pharmacologists and chemists to drive the emerging anti-metastasis theranostic technologies.
2. Advanced nanoparticle platforms for the construction of nanomedicines
In order to engineer individualized anti-metastasis nanomedicines, it is necessary to select suitable nanoparticle platforms that meet the special requirement. Here we highlight several promising nanoparticle platforms for constructing anti-metastasis nanomedicines, and summarize their key properties, nano-structural features and therapeutic and diagnostic advantages, as listed in Table 1.
Table 1.
Examples of nanoparticle platforms with advantages for constructing nanomedicines.
| Basic Elem. | Nanostructures | Material Properties | Therapeutic/Diagnostic Advantages |
|---|---|---|---|
| Organic |

|
High surface area; Small nano-size; Controllable biodegradation; Functionally integratable;20–23, 25 Difficult to purify or low yield |
High payloads of drugs and imaging agents;20, 21 Controlled drug release13, 16, 17 |
|
Soft, deformable; Biodegradable; Functionally integratable;29 Poor stability69 |
Multi-drug co-loading and co-delivery;70 Controlled drug release71–73 |
|
|
Biodegradable; Functionally integratable;40, 74 Poor stability75 |
Loading of hydrophobic drugs;35, 36 Controlled drug release36, 74 |
|
| Au |

|
Biochemically inert; Structurally expandable; Functionally integratable11, 66, 67 |
NIR-photothermal therapy;46, 48, 76, 77 Drug delivery, controlled release;78, 79 NIR-photothermal imaging;80 NIR-photoacoustic imaging;81 CT imaging;82 Two-photon luminescence imaging;83 Optical coherence tomography imaging84 |
| Fe3O4 |
|
Biodegradable57 | Magnetothermal therapy;58, 59 Magnetic targeting;60 MRI61–65 |
| Si, SiO2 |

|
Biodegradable; Biocompatible; Structurally expandable; Functionally integratable; High surface area; Large pore volume11, 85–89 |
Drug delivery, controlled release;85, 90, 91 Ultrasound imaging;92 Fluorescence imaging93, 94 |
| C |
|
Biochemically inert; Functionally integratable; High surface area95, 96 |
NIR-photothermal therapy;49–51 Drug delivery, controlled release;52–54 NIR-photoacoustic imaging;55 Fluorescence imaging56 |
Dendrimers are characterized by a type of 3-dimensional hyperbranched macromolecular nano-architectures derived from the bottom-up construction method. The hyperbranched structure of dendrimers has high internal surface area and plenty of active end groups, which allows the encapsulation or conjugation of a variety and number of functional molecules, such as hydrophobic and hydrophilic drugs, contrast agents and genes (Table 1). Typically, drug molecules (CPT, DOX, PTX, etc.) are conjugated with active end groups of dendrimers (PAMAM, PPI, PLys, etc.) to form prodrugs which are capable of stimuli-responsive (pH, redox, enzyme, etc.) release of drugs,13–19 while the high-proportion grafting of imaging molecules (Gd complexes, radioligands, etc.) within dendrimers favour the remarkable enhancement of their imaging performances.20–22 It is worth noting that several diagnostic and therapeutic nanomedicines based on polyamidoamine (PAMAM) dendrimers have been approved by FDA for clinical trials and even commercialized, such as VivaGel®, Priostar®, Stratus®CS AcuteCare™ and SuperFect®. Recently, Xie et al. coated two kinds of antibodies (anti-EpCAM and anti-sLeX) onto the surface of the G6 PAMAM dendrimer and then used the dual antibody-coated dendrimers to target the circulating tumour cells (CTCs). They demonstrated the enhanced efficiency of CTC capture and anti-metastasis effect by restraining CTCs and inhibiting their hetero-adhesion to blood vessels.23, 24 Moreover, dendrimers hold another important advantage in nano-size modulation. By controlling the generation, their sizes can be reduced to less than 3 nm in favour of their quick excretion in vivo, while bigger ones of 7–12 nm in diameter would be retained in the blood circulation system in favour of their blood pool imaging.25 In addition, their biodegradation and surface charge can also been tailored by selecting suitable monomers. Kaminskas et al. used lysine as monomer to synthesize a biodegradable polylysine dendrimer, and conjugated with DOX via an acid labile linker. The DOX-conjugated dendrimer was administrated by inhaling to improve the targeted therapy of lung metastases, and degraded in the lungs into low molecular weight fragments and then cleared into the urine.19, 26 On the other hand, there are two main drawbacks of dendrimers: 1) the product cannot be purified from side products by the divergent method (core-to-surface gradient condensation); 2) the yield of high-generation product is considerably low by the convergent method (fragment condensation). These lead to high costs and consequently obstruct their commercialization.
Liposomes are spherical self-closed vesicles composed of a lipid bilayer shell/membrane and an aqueous core. The unique structure allows liposomes to co-encapsulate and co-deliver multiple sized agents, especially hydrophilic plus hydrophobic drugs respectively encapsulated within the core and shell (Table 1).27 An excellent example is Myocet® (liposomal doxorubicin) approved in Europe and Canada for the treatment of metastatic breast cancer in combination with cyclophosphamide.28 Furthermore, liposomes are considerably flexible and deformable so that they can penetrate the 10–20 μm thick barrier comprised of stacked corneocytes.29 Additionally, liposomes are also subjected to disassembly, burst and biodegradation when suffering biomembrane and various stresses such as heating and pressure. On the other hand, the liposomal structure often needs to be consolidated to be stable enough for storage and in vivo delivery in order to meet clinical requirements.30 The balance between biodegradation and stability needs to be well mastered according to practical requirements. In addition, the outside surface of liposome can readily be modified with targeting molecules to improve the anti-metastatic efficacy.31–34 For example, chlorotoxin and cyclic RGD peptides were conjugated onto liposomes to target MMP-2 overexpressed on metastatic breast cancer and integrin αvβ3 for inhibiting bone metastases, respectively,32, 33 while mannose was conjugated to target and inhibit liver metastasis.34
Micelles are a kind of self-assembly aggregates of surfactant/amphiphilic polymer molecules driven by thermodynamics. Therefore, a micelle contains a relatively large hydrophobic region in the middle, allowing the loading and delivery of lyophobic drugs (Table 1).35–38 A representative example is poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles, which are biodegradable and capable of encapsulating a number of hydrophobic drugs.39, 40 Recently, Xu et al. constructed a new micelle with an amphiphilic copolymer of methoxy polyethylene glycol-S-S-vitamin E succinate (mPEG-s-s-VES, PSV), and used the micelle to load a large number of atorvastatin (a hydrophobic anti-metastatic drug) up to 50wt.% for anti-metastasis therapy.36 In addition to high loading capacity, the micelles also exhibited high drug encapsulation efficiency (99%) and redox-responsive drug release profiles, which together made contribution for enhancing intratumoural drug accumulation and blocking the lung and liver metastasis of 4T1 breast cancer.
Examples of organic nanoparticle platforms listed above for constructing nanomedicines hold several common key advantages: 1) possess well-defined biodegradability, biocompatibility, pharmacokinetics and pharmacodynamics; 2) several nanomedicines have been approved for clinical use; 3) easy surface modification and targeting molecule conjugation; 4) capable of controlled drug release and multifunctional integration. However, very few relevant anti-metastasis nanomedicines have been proven effective in the clinic, mainly due to: 1) lack of guidance of well-defined anti-metastatic strategies and the reasonable engineering of anti-metastasis nanomedicines; 2) developed organic nanoparticle-based nanomedicines have some limitations such as poor stability, drug leakage, etc.
Emerging inorganic nanoparticle platforms provide a good opportunity to overcome these issues and even play more unique roles in fighting against metastasis. Compared with organic nanoparticles, inorganic ones have relatively higher biophysicochemical stability. In addition, inorganic nanomaterials have many more unique physical properties, such as optical, electrical, magnetic and thermal properties and solubility/ionization ability, and also possess considerably varied nanostructures (Table 1), such as hollow (porous) nano-spheres, nano-tubes, nano-rattles, nanoflowers, nano-cages, nano-sheets, nano-rods, etc. These features are of remarkable values for expanding the structures, functions and performances of nanomedicines based on inorganic platforms. These advantages are introduced through several advanced inorganic nanoparticle platforms as follows.
Gold (Au) nanoparticles have two most attractive characteristics for biomedical applications: 1) the surface multivalent coordination; 2) the surface plasmon resonance (SPR) effect. The surface multivalent coordination with thiolates, carboxylates and amines allows facile surface conjugation with drug and targeting molecules to realize controlled drug release and targeted drug delivery.41–45 The SPR effect has been successfully used for photothermal therapy (PTT). By adjusting the surface/size/shape of Au nanoparticles, their plasmon absorption wavelengths can be tuned to the near-infrared (NIR) range, allowing NIR PTT (Table 1). Especially AuroLase® Au nanoshell (100–130 nm) developed by Halas and coworkers was approved for the PTT of human head and neck cancers by FDA in 2008, and was further authorized for PTT of human primary and metastatic lung tumours in 2012.46 Furthermore, the nanocages (30–50 nm) developed by Xia group have a smaller particle size and a hollow core–porous shell structure, and have therefore been utilized for controlled drug release and combined PTT–chemotherapy.47 Recently, we developed a kind of Au bellflower with high photothermal conversion efficiency (PTCA ~74%), which is higher than most other Au nanoparticles of different sizes and shapes (nanoshell 13%; nanorod 22%; hexapod 29.6%; nanocage 63%).48 Therefore we believe that the Au bellflower and other gold-based nanomaterials with high PTCA will have great potential for PTT. Besides therapy functions, Au nanoparticles also exhibit intriguing diagnosis performances in NIR-photoacoustic (NIR-PA), NIR-photothermal (NIR-PT), CT, two-photon luminescence (TPL) and optical coherence tomography (OCT) imaging, etc.41, 49–56
Superparamagnetic iron oxide nanoparticles (SPIONs) have several advantages as a nanomedicine platform: 1) excellent MRI T2 contrast; 2) the magnetothermal effect for hyperthermia therapy; 3) the superparamagnetism for magnetic targeting; 4) good biodegradability (acid soluble).57–65 Ferumoxides (Feridex®, 120–180 nm) and Ferucarbotran (Resovist®, 45–60 nm) were two dextran-coated SPIONs approved as MRI contrast agents for imaging liver lesions, but currently both are actually off the market, possibly owing to limited application on liver tissue and lack of tumour targeting. Subsequently, dextran-coated SPION Ferumoxtran-10 (Combidex®) with a smaller size (29.5 nm) and a longer plasma half-life (25–30 h) was designed to accumulate in the lymphatic system. Ferumoxtran-10 was therefore approved for the diagnosis of metastatic lymph nodes (LNs) in bladder cancer patients by MRI. Birkhäuser et al. recently demonstrated that ferumoxtran-10-mediated diffusion-weighted MRI reliably detected metastases in normal-sized LNs in at least two-thirds of their patients, which were undetectable by other imaging modalities.62 Recently, we prepared octapod SPIONs (edge length of 30 nm), which exhibit an ultra-high transverse relaxivity value (679.3±30 mM−1s−1), indicating that the octapod SPION is an much more efficient T2 contrast agent for in vivo MRI and small tumour detection in comparison with conventional ones and thus holds great promise for highly sensitive, early stage and accurate detection of cancer.63 In addition, a synergistically enhanced T1-T2 dual-modal contrast agent could be obtained by doping SPION with Gd in favour of highly accurate diagnosis with self-confirmation information.64, 65 It is worth noting that SPION can be integrated with other nanoparticle platforms such as mesoporous silica nanoparticles (MSNs), carbon and Au nanoparticles to construct multifunctional nano-structures for multimodal imaging, diagnosis and therapy (Table 1).11, 65–68
Mesoporous silica (or silicon) nanoparticles (MSNs) possess some unique advantages: 1) extensive mesoporosity with tunable pore size (2–20 nm) in favour of controlled drug release; 2) high surface area and large pore volume supporting high payload of drugs; 3) tunable particle size (10–1000 nm) and facile surface functionalization benefiting targeted delivery; 4) flexible nano-structure, excellent biocompatibility and biodegradability (Table 1).85, 97 The hollow MSN, in particular, has a super-high payload of drug, typically more than 1 gram drug per gram silica, and has therefore been used to enhance the loading capacity of various anti-metastatic drugs and genes, such as silibinin, DOX and siRNA, significantly improving their anti-metastatic efficacies.98–100 Furthermore, it is facile to integrate MSNs with other functional agents/nanomaterials into a single nanoparticle for multimodal diagnosis and therapy (Table 1).11, 64–68, 97 We recently used rattle-structured MSNs to construct a variety of multifunctional theranostic nanomedicines.10, 86, 89 Wiesner group developed silica-based Cornell dots (C dots, Cy5@silica-PEG) with a diameter of ~7 nm for imaging, and received the approval from FDA for a first-in-human clinical trial in 2010.101 Their initial studies claimed the biosafety of C dots as they exhibited in vivo stability, no toxic or adverse events and distinct renal excretion after injection for 2 weeks.102 These encouraging human data pave the way for clinical applications of other silica-based nanoparticles. Indeed, MSNs have entered preclinical studies in Shi and Tang groups desiring further advances toward clinical use.87, 88, 103
Carbon nanoparticles, including carbon nanotubes (CNTs), nano graphene oxide (NGO) and mesoporous carbon nanoparticles (MCNs), have two unique advantages for constructing nanomedicines: 1) super-high surface area for drug delivery and therapy; 2) efficient NIR optical absorption for NIR-photothermal (NIR-PT) imaging and therapy (Table 1). Owing to their unique molecular structure of alternant (only six-membered carbon ring) polycyclic aromatic hydrocarbon, CNTs and NGO can absorb a large amount of aromatic molecular drugs and many photosensitizers, such as DOX, CPT and porphyrins, via the non-covalent π–π stacking interaction, exhibiting a super-high drug loading capacity.54 Moreover, their NIR optical absorption property has also been developed for NIR-PT imaging and therapy, which only requires a low energy NIR laser to generate a relatively high thermal energy owing to high photothermal conversion efficiencies.49–51 Furthermore, functional molecules/nanoparticles such as SPION, MSN and Au nanoshell can be attached/coated on the surface of nano carbon materials to construct multifunctional nanoparticle platforms.95, 104, 105 In addition, hollow MCNs with better hydrophilic capability for biomedical application have also been developed.52, 53, 106
Each nanomaterial has its specific advantages and also disadvantages for constructing nanomedicines. Frequently, complex architectures, typically organic-inorganic nano-composite, are necessary to obtain more and better performances.107–112 For example, PEGylation of nanomaterials has proved to be an effective method for improving drug delivery and therapeutic efficacies; hyperbranched structures are designed to provide multifunctional groups for enriching properties of nanomaterials and integrating their advantages, such as encapsulation of insoluble drugs and imaging agents, cleavable linking of pro-drugs, conjugation of targeting moiety;107, 108 the coating of a lipid bilayer on the external surface of inorganic nanoparticle is used to integrate properties and advantages of liposomes;110, 111 the framework incorporation is used to adjust the biodegradability of nanomaterials, such as organosilicon.3, 112
3. Biological characteristics and mechanisms of tumour metastasis
It is well-known that tumour metastasis involves the completion of a complex succession of cytobiological events termed the metastasis cascade, which can be divided into no less than seven steps: 1) primary tumour cells invade the surrounding basement membrane and cross extracellular matrix (ECM) and stromal cells; (2) intravasate into blood/lymphatic vessels; (3) circulating tumour cells (CTCs) are transported through the vasculature; (4) arrest at distant tissue sites; (5) extravasate into the parenchyma of distant tissues; (6) adapt to survive in the foreign microenvironments of distant tissues; (7) seed, proliferate and colonize to generate metastases.113 In the view of macroscopically spatial alternation and key milestones, we reduce the metastasis cascade into three main stages: 1) pre-metastatic initiation (steps 1–2); 2) metastasizing dissemination (steps 3–4); 3) metastasized colonization (steps 5–7). In principle, the interruption of any one of these steps in the metastasis cascade will lead to the failure of metastasis, illuminating many trials to conquer tumour metastasis. Therefore aiming at these three main stages, we emphasize our train of thought for anti-metastasis: development of individualized anti-metastasis strategies to intercept each metastasis stage and fight pre-metastatic (Section 4), metastasizing (Section 5) and metastasized (Section 6) tumours. The following three sections will dissect and detail the biological characteristics of various stages of metastatic progress, and meanwhile propose a series of individualized anti-metastasis strategies by virtue of nanomedicine engineering.
4. Blocking and combating the initiation of metastasis from the primary tumour by nanomedicines
Anti-metastasis is much more difficult than anti-primary tumour as reflected by more powerful lethality of metastases and higher mortality of metastatic patients. Therefore, it is ideal to block and combat the initiation of metastasis from the primary tumour in the early stage, which involves the early theranostics of primary and metastatic tumours. Two essential but significant aspects need to be addressed: recognition of hallmarks for targeted therapy; high-resolution imaging for early diagnosis. Nanomedicines have proven to play important roles in these two aspects. Invasion as the first stage of metastasis therefore becomes the anti-metastasis frontier, and the primary tumour environment (TME) is not only educable to promote invasion by tumour cells but also reeducable to block invasion.114–116 Based on these two characteristics of invasion, we here propose two corresponding anti-metastasis nanomedicine strategies: 1) combating invasive cancer cells and 2) re-educating the primary TME, for blocking and combating the initiation of metastasis from the primary tumour by nanomedicines. These two strategies will be unfolded as follows.
4.1 Combating invasive cancer cells
In order to start the process of invasion, cancer cells must strengthen their interaction with surrounding cells and the extracellular matrix (ECM) for motility. Several cell adhesion proteins have been identified to be over-expressed on the surface of invasive cancer cells, including integrins (receptors mediating cell-ECM adhesions), cadherins (transmembrane proteins involved in cell-cell interactions), cell adhesion molecules (CAMs), etc.117 Their corresponding ligands, including small organic molecules, peptides, proteins, antibodies and aptamers, can be exploited as anti-invasion therapeutic agents (Cilengitide, CNTO 95, Etaracizumab, Vitaxin, S-247, just to name a few). These agents can be loaded into nanoparticles for efficient drug delivery and controlled release, and are also potential targeting heads which can be conjugated onto the surface of nanoparticles for targeted drug delivery.
A representative example is that RGD peptides as ligands of integrins are frequently used for construction of targeted nanomedicines. Especially integrin αvβ3 is highly expressed on activated endothelial cells, new-born vessels and some tumour cells, but is absent in resting endothelial cells and most normal organs, making it a suitable target for cancer therapy.120–124 Aiming at integrin αvβ3, we developed a wealth of targeted imaging and therapy nanomedicines by integrating various nanoparticle carriers/imaging agents with targeting ligands.118, 125–133 For example, SPIONs were coated with cRGDyK to construct an ultra-small SPION-cRGDyK nanomedicine (Fig. 1A1) with an overall diameter of ~8.4 nm (Fig. 1A2, hydrated ion diameter which is slightly bigger than the TEM size), and used it to specifically target to integrin αvβ3-rich tumour cells, which were readily tracked by MRI (Fig. 1A3);118 ferritin nanocages were used to load doxorubicin (DOX) and conjugate with RGD on the external surface (DOX@nanocage-RGD) for targeted drug delivery, showing a longer circulation half-life, higher tumour uptake, better tumour growth inhibition and less cardiotoxicity than free DOX.125 In addition, Murphy et al. used a liposome carrier to construct a targeted nanomedicine (DOX@Liposome-PEG/cRGDfK) by conjugating PEG and cRGDfK for targeting the integrin αvβ3-overexpressed R40P pancreatic tumour and loading DOX for chemotherapy (Fig. 1B1,2).119 The nanomedicine exhibited a considerably high tumour-targeted efficacy with limited distribution in normal tissues (Fig. 1B3), and the targeted delivery of DOX led to remarkable disruption of the pancreatic tumour vasculature (Fig. 1B4), and thus inhibited the growth of the primary pancreatic tumour as well as the metastasis toward the hepatic hilar lymph node (Fig. 1B5). Compared with free DOX drug, the integrin αvβ3-targeted drug delivery by liposome-based nanomedicine resulted in a 15-fold increase in anti-metastatic activity and minimal systemic toxicity.
Figure 1.
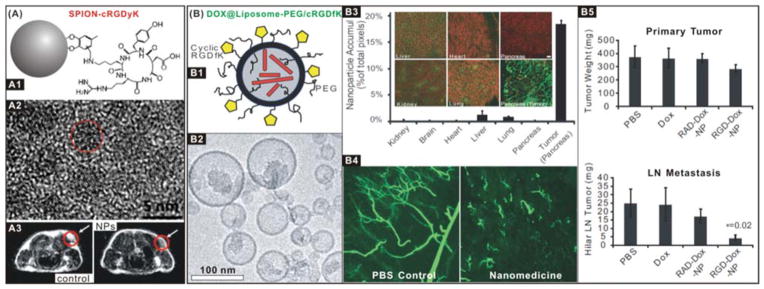
(A) SPION-cRGDyK nanomedicine for integrin αvβ3-targeted imaging and diagnosis: (A1) Schematic structure of ultra-small SPION-cRGDyK nanoparticle; (A2) HRTEM image of the iron oxide nanoparticle; (A3) MRI cross-section patterns of U87MG tumour mice treated with SPIONs without (control) and with (NPs) RGD targeting.118 Reproduced with permission from ref. 118, Copyright 2008 American Chemical Society. (B) DOX@Liposome-PEG/cRGDfK nanomedicine for integrin αvβ3-targeted therapy: (B1) Schematic structure of the DOX@Liposome-PEG/cRGDfK nanomedicine; (B2) TEM image of the nanomedicine; (B3) Distributions of the nanomedicine in R40P pancreatic tumour, where the green colour represents the nanomedicine binding; (B4) The vascular disruption in the mouse model treated with control and nanomedicine samples; (B5) Anti-primary tumour and anti-metastasis efficacies of the nanomedicine.119 Reproduced with permission from ref. 119, Copyright 2008 National Academy of Sciences, USA.
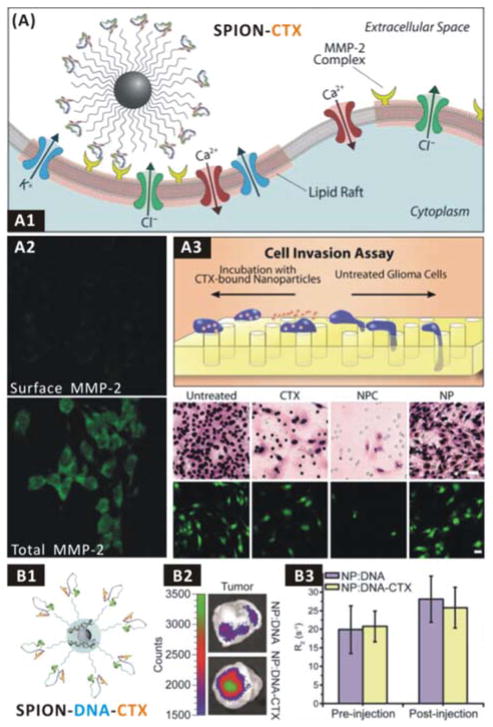
In order for invasive cancer cells to move forward, the ECM must be disintegrated to create room, which is achieved mainly through the secretion of proteinases, including matrix metalloproteinases (MMPs), ADAMs, cathepsins, etc.117 Likewise, their ligands are potential therapeutic agents when loaded in nanomedicines as well as targeting molecules when conjugated on the surface of nanomedicines for targeted drug delivery, and their enzymatic substrates can also be coated/linked on the surface of nanomedicines for proteinase-responsive drug release. Zhang group employed an anti-invasion therapeutic against gliomas, in which chlorotoxin (CTX, an inhibitor of MMP-2), as a targeting molecule was conjugated on the surface of SPIONs (SPION-CTX, Fig. 2A1).134 They found that SPION-CTX could target C6 glioma cells via binding to MMP-2 overexpressed on the cellular surface, and the multivalent binding promoted cellular internalization of a large portion of lipid rafts that contain surface-expressed MMP-2 and volume-regulating ion channels (Fig. 2A2), leading to a remarkably enhanced in vitro anti-invasion rate of ~98% compared to free CTX (~45%) (Fig. 2A3). Furthermore, they loaded reporter genes within SPION-CTX by surface physical adsorption (SPION-DNA-CTX) for targeted gene therapy (Fig. 2B1).135, 136 They found that SPION-DNA-CTX could enhance gene transfection efficiency in vitro (Fig. 2B2), but was lack of in vivo targeting ability as CTX did not affect the intratumoural accumulation of SPION-DNA-CTX (Fig. 2B3). In addition, the intratumoural localization of nanomedicines can be monitored using MRI as SPION is an excellent contrast agent for in vivo MRI tracking of cell invasion and migration.137
Figure 2.
(A) The SPION-CTX anti-invasion nanomedicine: (A1) Scheme of interaction between the SPION-CTX nanomedicine and MMP-2 on the cellular surface; (A2) Difference of surface and total MMP-2 expression of tumour cells after nanomedicine treatment; (A3) C6 glioma cell invasion inhibition effects in vitro.134 Reproduced with permission from ref. 134, Copyright 2009 Wiley-VCH. (B) The SPION-DNA-CTX anti-invasion nanomedicine: (B1) Structure of SPION-DNA-CTX; (B2) Transfection of GFP-encoding DNA to C6 xenograft tumours using the SPION-DNA-CTX anti-invasion nanomedicine; (B3) Nanomedicine delivery to C6 xenograft tumours monitored by MRI.135 Reproduced with permission from ref. 135, Copyright 2010 American Chemical Society.
In addition, the secretion of MMPs for focally degrading the ECM is mainly achieved by invadopodia, which are actin-based membrane foot-like protrusions expressed on invasive tumor cells. Invadopodia have proven to pave a way for the directional movement (intravasation and extravasation) of invasive tumor cells through focal matrix degradation. Invadopodia are therefore identified as a therapeutic target for anti-metastasis. Some invadopodia inhibitors, such as imatinib mesylate, sunitinib (PDGFR inhibitors), trastuzumab, lapatinib (HER-2 inhibitors), dasatinib, saracatinib, bosutinib (SRC inhibitors), have been developed to block tumor invasion and metastasis.138–140 However, invadopodia inhibitors have a distinct shortcoming as they work on prophylactically blocking the further spread of metastasis rather than the growth of formed metastasis.141 Therefore, to combine anti-invadopodia therapy with other therapies, such as chemotherapy, will be more effective for anti-metastasis. In this way, we predict that nanomedicines will play an active role in the targeted co-delivery of multiple drugs (such as invadopodia inhibitor plus chemotherapeutic drug) for improving anti-metastasis therapy efficacy.
During the invasion, invasive cancer cells must be able to squeeze themselves through narrow spaces of the reconstructed ECM, which is generally achieved by altering the osmotic balance between the cancer cells and extracellular space through aquaporins and ion transporters, such as sodium-potassium-chloride co-transporter isoform-1 (NKCC1), chloride channel-3 (ClC-3), transient receptor potential cationic channel (TRPC6), etc.117 Moreover, the activation of transcription factors mediates cell invasion by direct interaction or indirect signal transduction, turning on the invasive phenotype. Several transcription factors, including NFATs (nuclear factor of activated T cells), PAR-1 (protease-activated receptor 1), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and STAT3 (signal transducer and activator of transcription 3), have been identified to be involved in promoting the expression of pro-invasion proteins.117, 142, 143 Typically, NFATs can be activated by TRPC6 calcium ion channel, and then promote the expression of a variety of pro-invasion molecules and also facilitate angiogenesis and lymphangiogenesis. Both cyclosporin A (CsA) and FK506 drugs (two calcineurin inhibitors) can inhibit the pro-invasion function of NFATs by preventing NFATs from entry into the nucleus, but also exhibit severe toxic side effects in cancer therapy owing to the lack of targetability. In this context, versatile nanomedicines are therefore proposed to mediate the targeted delivery of these drugs to reduce their toxic side effects as well as to enhance their drug efficacies.
Here we summarize the evolution of invasion-targeted nanomedicines, as illustrated by Fig. 3. In the first stage, single function nanoparticle is integrated with targeting molecules to realize a simple aim of invasion-targeted imaging or therapy (Fig. 3A). The therapy effect of nanomedicines results from the multivalent binding and cellular internalization of lipid rafts containing invasion-associated molecules, and thus the target points of nanomedicines are limited to invasion-associated molecules on the surface of cancer cells. The use of nano-carriers to encapsulate a therapeutic drug can further realize the targeted drug delivery (Fig. 3B), which has indeed enhanced the therapy efficacies of nanomedicines. Furthermore, we propose to further enrich and strengthen alternative anti-invasion strategies by integrating a wide variety of therapeutic drugs and targeting molecules with multifunctional nano-carriers (Fig. 3C). A prospective anti-invasion nanomedicine will possibly hold multi-target heads and load multi-drugs for highly efficient imaging and therapy. In such a way, nanomedicines can be designed to hierarchically and sequentially target to vascular-to-cell-to-nuclear and deliver various kinds of drugs and imaging agents, such as genes, chemotherapeutants, antibodies, and even secondary targeting molecules.144
Figure 3.
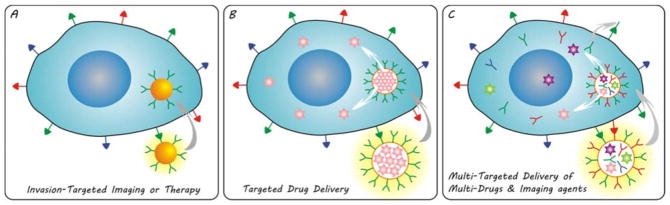
Evolving invasion-targeted nanomedicines: (A) single function nanoparticle-based nanomedicine for invasion-targeted imaging or therapy; (B) multifunctional nanoparticle-based nanomedicine for targeted drug delivery; (C) versatile nanomedicine for multi-targeted delivery of multi-drugs and imaging agents.
In addition to targeting molecules and therapeutic drugs, nanoparticle platforms for constructing anti-invasion nanomedicines need to be thought over as some nanoparticles could affect the functions of invasive cells. Zhao et al. investigated the anti-invasion activity and mechanism of gadolinium metallofullerenol nanoparticles (f-NPs) using a tissue invasion animal model (JF305 human pancreatic tumour xenograft model).145, 146 They found that f-NPs not only suppressed the expression of MMPs (MMP-2 and MMP-9) via an exocite interaction, but also inhibited their activity by intensive binding with residues near the ligand-specific loop S1′, thereby leading to significantly less metastasis to the ectopic site from the invasive primary tumour. Alili et al. found that dextran-coated cerium oxide nanoparticles exhibited a cytotoxic, pro-apoptotic and anti-invasive capability against A375 melanoma cells, but no apparent toxicity against stromal cells in vitro and in vivo.147, 148 They attributed this selective anti-invasion capability to a selectively pro-oxidative property of cerium oxide nanoparticles as they found that the intracellular ROS level in tumour cells were increased by twofold but no increase was detected in normal cells. The selective pro-oxidative effect of cerium oxide nanoparticle makes it promising for anti-metastasis therapy. Moreover, cationic functionalized fullerene and hydroxyapatite nanoparticles also showed in vitro anti-invasion activity by interfering with the activities of MMP-2 and MMP-9.149, 150 Liu et al. discovered that small citrate-capped Au nanoparticles (Au-NPs) exhibited in vitro cytotoxic and pro-invasive effects against some types of tumour cells, which depend on the particle size of Au-NPs and cell types. They investigated the effect of Au-NP size on the invasion activity of lung cancer cells and found that 5-nm and 10-nm Au-NPs significantly promoted the invasion of cancer cells but larger Au-NPs (20 and 40 nm) did not. Furthermore, they associated the increased invasion activity of lung cancer cells with the upregulated expression of MMP-9 and intercellular adhesion molecule-1 (ICAM-1) by small Au-NPs.151
4.2 Re-educating the primary tumour microenvironment (TME)
Besides tough elimination of cancer cells, gentle re-education of the primary tumour microenvironment (TME) is another alternative strategy to block the initiation of metastasis from the primary tumour. The TME involves cancer cells, a variety of stromal cells, their exosomes (including cytokines and chemotactic factors), etc. Therein, stromal cells contain endothelial cells, stromal fibroblasts and bone marrow-derived cells (BMDCs) including macrophages, myeloid-derived suppressor cells (MDSCs), TIE2-expressing monocytes (TEMs) and mesenchymal stem cells (MSCs). A dynamic interplay between cancer cells and stromal cells is well recognized: tumour cells hijack/educate normal stromal cells to become bad.152–154 For example, normal endothelial cells, fibroblasts, macrophages and lymphocytes are educated into tumour-associated endothelial cells (TAECs), tumour-associated fibroblasts (CAFs), tumour-associated macrophages (TAMs) and tumour infiltrating lymphocytes (TILs) in the TME, respectively, which not only lose their defence functions but also support tumour progression and metastasis.155
In the past few years, most of studies on anti-metastasis have focused on the inherent migratory capability of cancer cells. However, the therapeutic strategies directly aimed at cancer cells frequently led to multidrug resistance (MDR) owing to intertumoural and intratumoural heterogeneities, which actually fosters the metastases. By comparison, stromal cells in the TME have less heterogeneity and higher genetic stability, and have recently found to be vitally important in promoting tumour invasion and metastasis. The TME-targeted anti-metastasis strategy may therefore have more probability to avoid the generation of genetic variation and MDR and obtain more stable therapeutic outcomes, consequently garnering specific attention recently.156 Due to some successful stories about normalization of cancer cells by modulating/reprogramming the microenvironment, a concept of “re-education of the TME” was proposed as a new anti-metastasis strategy to fully reverse the pro-tumourigenic TME and recreate a suppressive microenvironment, which is expected to effectively block the initiation of metastasis from the primary tumour.152
As demonstrated in Fig. 4, a wide range of therapeutic agents have been exploited to battle the initiation of metastasis from the primary tumour by re-educating the primary TME. Based on the pro-metastatic mechanisms of tumour-associated stromal cells (TASCs), three leading reeducation strategies were summarized as anti-angiogenesis and anti-lymphangiogenesis,157, 158 repolarization of TAMs115, 159 and immunomodulation,160–166 and some relevant reeducation agents were shown in Fig. 4.
Figure 4.
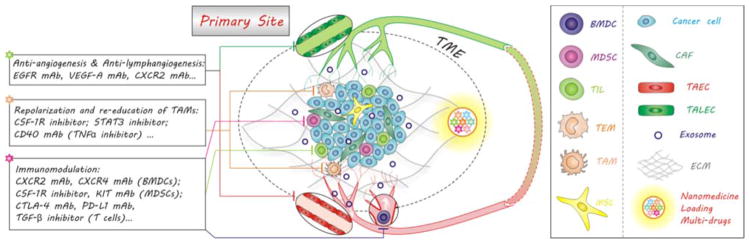
Re-education of the primary tumour microenvironment (TME) with nanomedicines to block the initiation of metastasis. Nanomedicines can be engineered to co-load multiple drugs with different functions and co-deliver them to tumour for synergetic re-education of the TME containing cancer cells, stromal cells and their exosomes by various routes including anti-angiogenesis, anti-lymphangiogenesis, repolarization of TAMs and immunomodulation.
The normalization of tumour vasculature, solid stress and extracellular matrix structure can make contributions to normalizing the TME for advancing delivery of anti-cancer nanomedicines.167–169 Jain et al. found that anti-angiogenesis therapy by combined use of antiangiogenic agents such as anti-VEGFR2 antibody can transiently normalize tumour vasculature and thus enhance the size-dependent tumour delivery efficiencies of molecular/nano-medicines, improving the therapy outcome.170–173 Besides, targeted NO gas therapy can also normalize the tumour vasculature, and thus improved tumour oxygenation and response to radiation treatment.174, 175
The repolarization or re-education of TAMs can be achieved by inhibiting CSF-1R, TNFα and STAT3.115, 159 For example, Zhang et al. used liposomal nanoparticles to encapsulate an STAT3 inhibitor (hydrazinocurcumin), and effectively delivered the drug into solid tumours to suppress STAT3 activity and re-educate TAMs.176 This formula successfully reversed the phenotype of TAMs and regulated the crosstalk between tumour cells and TAMs through inhibiting STAT3 signalling, inhibiting breast tumour proliferation, angiogenesis and pulmonary metastasis in vivo.
Moreover, the recruitment and expansion of immune cells can be blocked by inhibiting several critical cytokine axes: CXCR2, CXCR4, CSF-1R and KIT.177–179 As mentioned above, therapeutic agents such as antibodies and antagonists can be delivered for therapy using nano-carriers, and can also be developed as targeting heads to mediate targeted delivery of drug molecules and imaging agents. For instance, we have successfully radiolabeled CXCR4 antagonist peptides to track CXCR4 expression on metastatic tumour models by PET.180–183 He et al. also conjugated an anti-CXCR4 mAb onto SPION for MRI of pancreatic cancer.184 Chittasupho et al. conjugated a CXCR4 antagonist (LFC131) on PLGA nanoparticles and loaded anti-cancer drug DOX to construct a targeted anti-cancer nanomedicine, which enhanced drug efficacy and lowered non-specific cytotoxicity to normal cells.185
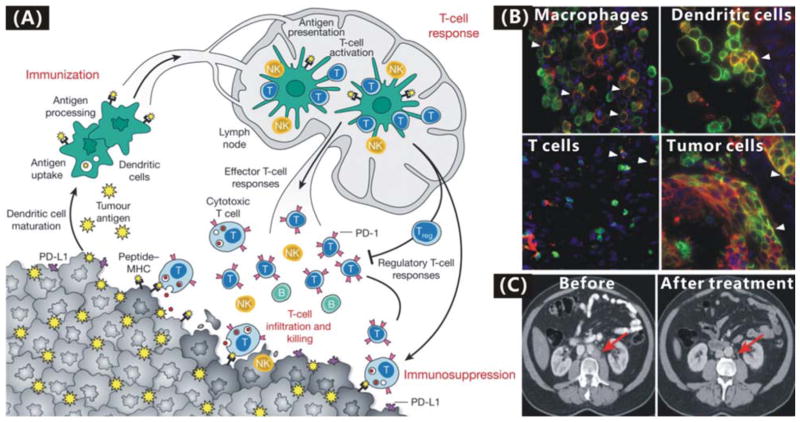
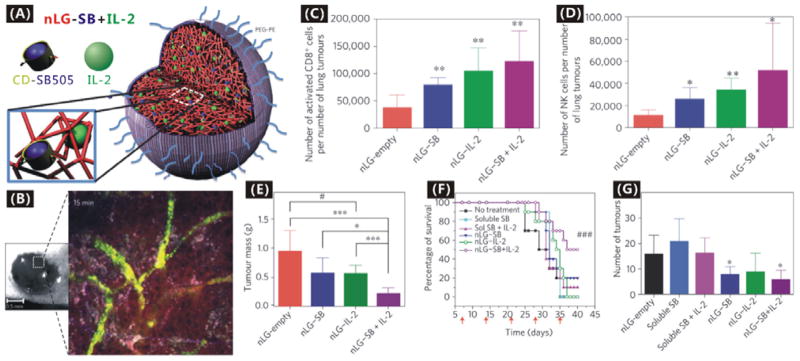
Additionally, the anti-PD-L1 immunotherapy has attracted much attention lately.160–165 Broad expression of PD-L1 (the predominant ligand for programmed cell death-1 receptor) on many types of tumour cells and lymphocytes including T cells, B cells, dendritic cells and macrophages in the TME mediates the immunosuppression effect (Fig. 5A).166 MPDL3280A (a human Fc-optimized anti-PDL1 mAb) was engineered to target PD-L1 for inhibiting the binding of PD-L1 to PD-1 and freeing/reversing tumour-infiltrating cytotoxic T lymphocytes (CTLs) in the TME to kill tumour cells locally (Fig. 5B).162, 163 Most importantly, MPDL3280A exhibited a few, low-grade toxicities and adverse events but high tolerability in the treatment of metastatic bladder and lung cancers, and consequently received FDA’s Breakthrough Therapy designation in June 2014.162, 163 It is expected that the immunotherapy effect of anti-PDL1 mAb can be further improved and its toxic side effects can also be reduced by targeted drug delivery with nanomedicines, as evidenced by some other immunotherapeutic nanomedicine formulas. For example, Park et al. constructed an immunotherapeutic nanomedicine (nLG-SB+IL-2) by using liposome (nanolipogels, nLGs) to co-encapsulate a hydrophobic transforming growth factor-β (TGF-β) inhibitor SB505124 and a hydrophilic interleukin-2 (IL-2), as shown in Fig. 6A.186 The nLG-SB+IL-2 formula can deliver these two drugs in a sustained fashion to the TME of a mouse model of B16/B6 melanoma (Fig 6B), activated both CD8+ T cells and natural killer (NK) cells in the TME (Fig. 6C and D), and thus resulted in enhanced immunotherapy efficacy compared with free drugs and single drug-loaded nanoformulas (Fig. 6E). Moreover, drug toxicities to normal tissues were remarkably reduced as indicated by the increase in the survival rate (Fig. 6F), and metastasis was also suppressed to a certain extent (Fig. 6G).
Figure 5.
(A) The anti-PD-L1 immunotherapy of cancer by blocking PD-L1/PD-1 pathways in the TME.166 Reproduced with permission from ref. 166, Copyright 2011 Nature Publishing Group. (B) Co-localization of PD-L1 (red) and markers (green) of tumour-infiltrating immune cells and tumour cell by immunofluorescence staining.163 Reproduced with permission from ref. 163, Copyright 2014 Nature Publishing Group. (C) CT images of a bladder cancer patient before and after the 2-cycle treatment with MPDL3280A had almost complete remission.162 Reproduced with permission from ref. 162, Copyright 2014 Nature Publishing Group.
Figure 6.
(A) Construction of nLG-SB+IL-2 nanomedicine by co-encapsulating SB505124@cyclodextrins and IL-2 in PEGylated liposome; (B) Demonstration of nanomedicine (green) and released drug (red) diffusions within the subcutaneous melanoma tumour by time-resolved intravital two-photon laser scanning microscopy after intravenous injection; (C) Absolute number of CD8+ T cells present in per number of tumours; (D) Absolute number of NK cells per number of tumours; (E) Tumour masses after treatment for seven days; (F) Survival rates of mice after treated for seven days; (G) Number of metastatic tumours (0.5–2 mm in diameter) in lungs of mice 14 days after initiation of treatment.186 Reproduced with permission from ref. 186, Copyright 2012 Nature Publishing Group.
Although a wide range of therapeutic agents are already available for educating the TME, a single agent is limited to work on only a small part, not all of TASCs in the TME. Therefore, the combined administration of multiple TME-educated drugs will be necessary for TME therapy. Moreover, high attention should be paid to the combination of TME therapy and tumour cell-directed therapies such as chemotherapy and radiotherapy, which may yield more robust outcomes than either therapy alone.187 Based on this rationale, nanoparticle platforms (Table 1) holding high surface areas and cavity volumes, especially hollow core-mesoporous shell nanoparticles (hMSNs), can be utilized to encapsulate a variety and number of drug molecules.7, 86, 188, 189 Moreover, sizes of hollow pore, mesoporous shell and mesopores are tunable in favour of controlling the whole particle size, the drug loading capacity and the drug release rate.190, 191 Furthermore, a therapeutic nano-core(s), such as Au, Pd and Fe3O4 nanoparticles, can be facilely integrated into an hMSN particle to construct a rattle-structure. In this way, other therapy modules such as thermotherapy, radiotherapy and HIFU therapy can be further combined with TME therapy and chemotherapy.97 In addition, multifunctional nanomedicines can be engineered to realize the targeted delivery and controlled release of therapeutic drugs to the TME, which is important to reduce toxic side effects of drugs and enhance drug efficacies.
5. Intercepting circulating tumour cells by nanomedicines for diagnosis and therapy
5.1 Intercepting CTCs in the blood system
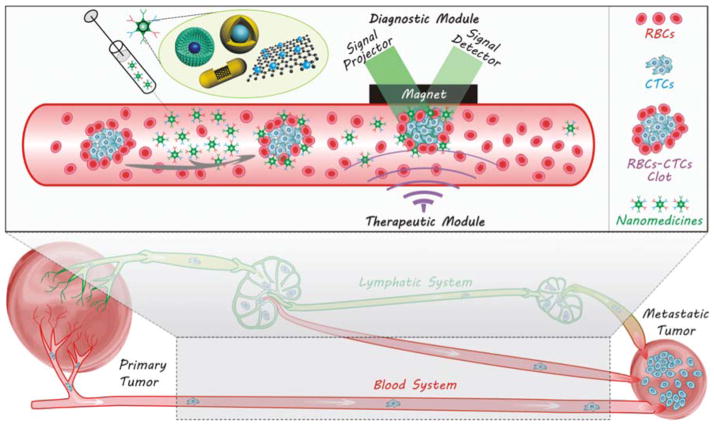
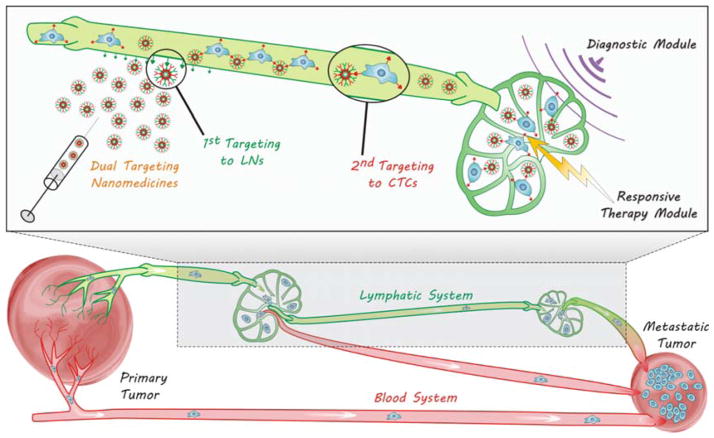
After detaching from primary tumours, metastatic tumour cells will travel a long way in blood and/or lymphatic circulation systems before lodging at a new location, which are therefore named circulating tumour cells (CTCs). CTCs have two main circulating routes: circulation in blood vessels (BV) and lymphatic vessel-to-blood vessel (LV-to-BV) circulation, including sentinel lymphatic node-to-blood vessel (SLN-to-BV) circulation and distant lymphatic node-to-blood vessel (DLN-to-BV) circulation, as demonstrated in Fig. 7. Aiming at these routes, several strategies by virtue of nanomedicine engineering are correspondingly proposed to intercept CTCs en route for diagnosis and therapy of metastasis according to the characteristics of routes and the CTCs.
Figure 7.
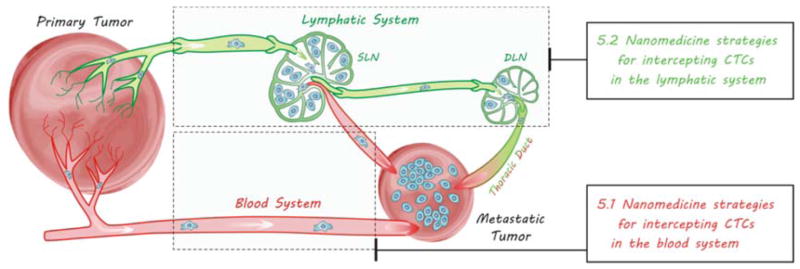
Metastasis routes of CTCs via two circulation systems.
Most of CTCs would be mechanically arrested en route by the capillary bed of distant organs, and the liver, lung and bone therefore become the common sites of tumour metastasis.192 These common sites of intensively arresting CTCs provide the potential windows to kill them. In addition, the escaped CTCs from the capillary bed could also survive partly, leading to the various sites of metastases according to the metastatic heterogeneity.193 It was found that less than 0.01% of CTCs can survive during the blood circulation to produce metastases, but they indeed account for more than 90% cancer-related deaths.194 Therefore, to find effective approaches to intercept and kill these surviving CTCs is important to block metastasis.
In order to intercept CTCs, the identification of their molecular characteristics is of critical importance. So far, some major advances have been made in this aspect. Some over-expressed antigens on CTCs derived from various common tumours have been identified and even quantified, and many corresponding antibodies have also been developed, as summarized in Table 2. Therein, epithelial cell adhesion molecule (EpCAM) tends to be over-expressed on most types of CTCs, and therefore becomes the most versatile target. Furthermore, these important immunologic features can be well utilized for immuno-mediated imaging, diagnosis, separation and therapy of CTCs by combination with nanotechnology.
Table 2.
Immunologic specificity and sensitivity of antigens over-expressed on CTCs derived from various cancers, as indicated by the number of +.
| Breast cancer | Lung cancer | Liver cancer | Pancreatic cancer | Ovarian cancer | Colorectal cancer | Prostate cancer | |
|---|---|---|---|---|---|---|---|
| EpCAM | +++ | ++ | + | + | + | ++ | +++ |
| HER2 | +++ | ||||||
| EGFR | + | + | + | ||||
| CK | + | ++ | ++ | + | |||
| CEA | + | + | + | ++ | |||
| MUC-1 | ++ | + | ++ | ++ | + |
We summarized a variety of diagnosis and therapy methods (Table 3), all of which are mediated by immunoreactions on CTCs. Among them, the most mature methodology is probably magnet separation-based diagnosis/detection, which is addressed by conjugation of specific antibodies on magnetic nanoparticles. The CellSearch™ based on ferrofluid nanoparticle-anti-EpCAM antibody conjugate is a most successful commercial product for CTC detection/diagnosis, and is also the only system approved by the US FDA for clinical practice in metastatic breast, colorectal and prostate cancer. The CellSearch™ has high sensitivity and reproducibility for CTC diagnosis, and also enables CTC quantification, which benefits from the relatively high sensitivity and specificity of conjugated anti-EpCAM antibody to CTCs. However, one major limitation of the CellSearch™ is potential false-negativeness owing to the heterogeneity of EpCAM expression between tumour subtypes and the down-regulation of EpCAM during epithelial-to-mesenchymal transition (EMT).206–208 An optimized combination method is introduced to further enhance the accuracy of CTC diagnosis by combination of multiple antibodies on a single nanoparticle.204, 209, 210 Additionally, the speed of CTC separation can be improved by using nanoparticles of stronger magnetism.197, 203, 204
Table 3.
Immuno-mediated diagnosis and therapy of CTCs through multifunctional nanomedicines.
| Aims | Approaches | Multifunctional Nanomedicines | Ref. |
|---|---|---|---|
|
| |||
| Ex vivo Diagnosis | Fluorescence imaging | Ferrofluid nanoparticles-anti-EpCAM antibody (CellSearch™); | 195, 196 |
| Fe@C-anti-EpCAM antibody; | 197 | ||
| SPION-anti-HER2 antibody; | 198 | ||
| RITC-silica nanoparticles-MUC1 monoclonal antibody | 199 | ||
|
| |||
| Fluorescence and dark-field imaging | SPION@Au nanoshell-anti-EpCAM/anti-HER2/anti-EGFR/anti-CK antibodies | 200 | |
|
| |||
| Photoacoustic imaging | CNT@Au nanoshell-folate | 201 | |
|
| |||
| SERS assay | Au nanoparticles-anti-EGFR antibody | 202 | |
|
| |||
| High-resolution X-ray imaging | Au nanoparticles; | 203 | |
| Bi nanoparticles-folate | 204 | ||
|
| |||
| In vitro Therapy | Photothermal therapy | Au@Si nanowires-anti-EpCAM antibody | 205 |
|
| |||
| X-ray irradiation therapy | Bi nanoparticles | 204 | |
In addition to magnetic nanoparticles for magnet separation-based diagnosis, some other functional nanoparticles, mainly inorganic ones such as Au, Bi, Si and silica nanoparticles, have also been used to realize photoacoustic, fluorescence, SERS and high-resolution X-ray imaging as well as photothermal and X-ray irradiation therapies by virtue of immunoreactions. Au nanoparticles, including nanorods, nanoshells and nanoroses, have been developed for synchronous photoacoustic, SERS and high-resolution X-ray diagnosis and photothermal therapy of CTCs, while Bi nanoparticles can be used for synchronous X-ray diagnosis and irradiation therapy of CTCs. Inspired by general anti-cancer strategies against primary tumours, we propose the multi-modal combination diagnosis and therapy strategy for intercepting and killing CTCs by proper design of multifunctional nanoparticle platforms, as illustrated in Fig. 8. For example, the rattle-structured nanotheranostic systems can be facilely constructed by integrating mesoporous shell with functional magnetic/fluorescent/heavy core (e.g. Au/Ag nanorod, Pd nanosheet, GO nanosheet, UCNP and Fe3O4 nanoparticle, etc.), and are used to combine a variety of diagnosis and therapy modes, such as photothermal/magnetothermal/radiosensitizing/chemical/photodynamic/HIFU therapies and MRI/fluorescent/photoacoustic/CT diagnoses.97
Figure 8.
Multi-modal nanotheranostics of CTCs in the blood circulation system with multifunctional targeting nanomedicines. Multiple antibodies as listed in Table 2 can be selected to co-conjugate on the nanomedicine to enhance the accuracy of individualized CTC detection. Multifunctional nanoparticle platforms, especially rattle-structured ones, can be used to realize in vivo theranostic of CTCs by combining a variety of diagnosis and therapy modes, such as photothermal/magnetothermal/radiosensitizing/chemical/photodynamic/HIFU therapies and MRI/fluorescent/photoacoustic/CT diagnoses.
Furthermore, most of the existing detection and separation methods involve ex vivo operation, while the therapy of CTCs needs in vivo administration. Therefore, two separate operations lose the efficacy and increase the complexity. However the above-proposed nanotheranostic systems will be able to monitor the outcome of CTC therapy in vivo, in situ and in real time (Fig. 8), and greatly enhance the diagnosis and therapy efficacies. Moreover, when the diagnosis result is found to be positive, the therapy function can be designed to be triggered by external stimuli, such as magnetic/photo/radio fields (Fig. 8).
During the engineering of CTC nanotheranostics, several key factors must be considered. For example, 1) blood flow could influence the detection and enrichment of CTCs, and nanoparticles therefore have to be sensitive enough to be captured by the detector and/or collector; 2) to avoid the non-specific capture of reticuloendothelial system, “a stealthy coat” such as PEG possibly needs to be dressed; 3) in the case of CTC enrichment for nanotheranostics, pre-enrichment of nanoparticles needs to be avoided before nanoparticles seize CTCs.
One major advantage of immune-mediated methodology is that the CTC-identifying efficacy and versatility of nanomedicine can be improved by combining multiple antibodies on a single but multifunctional nanoparticle, which depends on the existing knowledge of molecular characteristics of CTCs and nanotechnology. Both false-positive (the wrong identification of epithelial-like non-tumour cells as tumour cells) and false-negative (the wrong identification of tumour cells as non-tumour cells) may still occur, owing to the lack of effective discrimination of epithelial markers between epithelial-like non-tumour cells and tumour cells and the metastatic heterogeneity of tumours which may inconstantly express given markers. This conveys the present challenge in the recognition of tumoural markers with high sensitivity (true-positive) and specificity (true-negative).208 In addition, the proportion of CTCs is extraordinarily small (only several CTCs per 1 mL whole blood), and the current isolation technologies cannot obtain sufficient numbers of CTCs, limiting the understanding of their biological features. Recently, Tseng et al. developed a new type of nanostructure-embedded microchips to improve the isolation efficiency of CTCs.211 They pioneered a unique concept of “NanoVelcro” cell-affinity substrates coated with anti-CTCs antibodies. The first-generation NanoVelcro chip composed of a silicon nanowire substrate (SiNS) showed a higher sensitivity compared with CellSearch™.212, 213 In conjunction with the use of the laser micro-dissection technique, second-generation NanoVelcro chips (NanoVelcro-LMD) realized single-CTC isolation.214, 215 By grafting thermoresponsive polymer brushes onto SiNS, third-generation NanoVelcro chips have demonstrated the capture and release of CTCs at 37 °C and 4 °C, respectively, allowing for the rapid purification of CTCs.216
5.2 Intercepting CTCs in the lymphatic system
In addition to the blood system, the lymphatic system is another important route of metastasis. The absence of hepatic first-pass effect leads to easier survival and dissemination of CTCs in the lymphatic system. The over-expression of some lymphangiogenesis factors such as VEGF-C by tumour cells causes the expansion and lymphangiogenesis of lymphatic networks around tumours, facilitating the spread of tumour cells towards the lymphatic system.217–220 The drainage of angiogenesis/lymphangiogenesis factors induces angiogenesis/lymphangiogenesis in SLNs and DLNs,221 forming a LV-to-BV route for metastasis, as illustrated in Fig. 7. Most of CTCs entering the lymphatic system mainly accumulate in the SLN since the lymphatic system lacks a central pump, while a small number of CTCs can also spread to DLNs, even to the blood system (Fig. 7). Owing to the interconnections of blood and lymphatic systems, CTCs are frequently synchronously disseminated into these two systems.222 Therefore to intercept and kill CTCs in the lymphatic system is also an important aspect of anti-metastasis.
Organic nanoparticles such as emulsions, liposomes, polymeric nanoparticles and nano-capsules can play an important role in intercepting and killing CTCs in the lymphatic system due to their natural lymph-targeting properties, e.g. through phagocytosis as a major uptake mechanism.223–225 In order to increase the lymph-targeting efficiency, the decrease of particle size and various surface modifications to nanoparticles have proven to be effective, e.g. PEGylation, galactosylation, immunization, surface coating with poloxamines/poloxamers/polyethyleneglycols, etc.226–231 Recently, Kaminskas et al. used PEGylated polylysine dendrimers (Fig. 9A1) to target the lymphatic system for delivering a model chemotherapeutic (methotrexate, MTX) through subcutaneous administration.232 The PEG-MTX-conjugated nanomedicine D-MTX(OH) was easily absorbed from the subcutaneous injection site via the lymph and about 30% dose/g node was retained in sentinel lymph nodes (Fig. 9A2), while free MTX alone was not absorbed into the lymph. Targeted delivery of chemotherapeutic MTX killed about 70% of the CTCs in the lymph (Fig. 9A3), and also inhibited the growth of lymph node metastases (Fig. 9A4). However, a large proportion of nanoparticles in other normal lymph nodes and a large number of macrophages were also killed non-specifically, which was possibly a result of the lack of targetability to the CTCs in the lymph and might cause potential toxicity. In order to diagnose SLN metastasis, we developed a MSN-based nanomedicine (Dye@MSN@Gd@64Cu) with triple-modal imaging capability by embedding/conjugating near-infrared dye ZW800, Gd-based contrast agent (Gd-DTPA) and positron-emitting radionuclide 64Cu within the MSN (Fig. 9B1).233 The constructed nanomedicine can visualize tumour draining SLNs in a 4T1 tumour metastatic model up to 3 weeks (Fig. 9B2) through near-infrared fluorescence (NIRF), magnetic resonance (MR) and positron emission tomography (PET) (Fig. 9B3–5). More importantly, tumour SLNs (T-SLNs) showed significantly stronger signals than normal ones (N-SLNs) at all the examined time points after subcutaneous or intravenous injection of nanomedicine (Fig. 9B3,5), indicating the preferred enrichment of nanomedicine in the T-SLNs. However, the injected nanomedicines also entered into the blood circulation and were subsequently captured by the liver, possibly owing to absence of adhesion/targetability to the CTCs in the SLNs.
Figure 9.
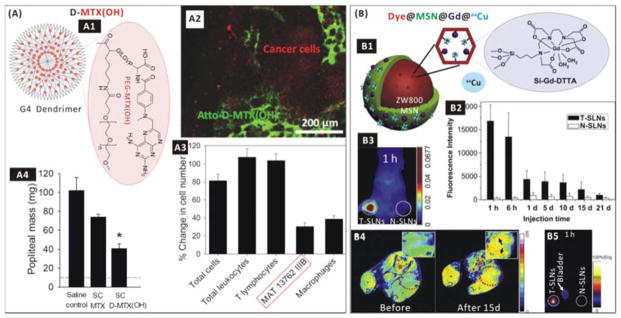
(A) Lymph-targeted drug delivery using the dendrimer-based nanomedicine. (A1) Construction of the D-MTX(OH) nanomedicine; (A2) Confocal fluorescent image of a popliteal lymph node bearing MAT metastases (red-labelled) 3 days after subcutaneous injection of green-labelled D-MTX(OH) into the inner heel of rats; (A3) Cytotoxicity of the D-MTX(OH) nanomedicine to lymph node cells 8 days after treatment of rats bearing popliteal lymph node-resident metastases of MAT 13762 IIIB carcinoma; (A4) Anti-metastasis efficacy of the nanomedicine against popliteal lymph node-resident metastases of MAT 13762 IIIB carcinoma.234 Reproduced with permission from ref. 234, Copyright 2014 Elsevier. (B) Imaging and diagnosis of the SLNs of metastasis. (B1) Construction of the Dye@MSN@Gd@64Cu nanomedicine; (B2) Retention of the nanomedicine in T-SLNs and N-SLNs of a 4T1 tumour metastatic model after subcutaneous administration; (B3) NIRF imaging, (B4) MRI and (B5) PET imaging of SLNs in the 4T1 tumour metastatic model after injection of the nanomedicine.233 Reproduced with permission from ref. 233, Copyright 2012 Elsevier.
The existing strategies of nanomedicine for anti-CTCs in the lymphatic system are all based on lymph-targeted delivery of anticancer drugs and imaging agents. However, the lymph-targeted delivery of anticancer drugs cannot guarantee no toxic side effect resulted from the non-specific drug release outside of CTCs or SLNs. Therefore, we propose a dual targeting strategy to construct LN- and CTC-targeted nanomedicines by conjugating specific antibodies or ligands as targets of CTCs, as illustrated in Fig. 10. Such LN- and CTC-targeted nanomedicines are expected to improve the sensitivity, specificity and efficacy of anti-CTCs in the lymphatic system. Moreover, the multi-modal combination nanotheranostic strategy mentioned in Section 5.1 may also be adequate for intercepting and killing CTCs in the lymphatic system besides those in the blood system with multifunctional nanoparticles. In addition, inorganic nanoparticle platform-based anti-CTC nanomedicines appear to be more suitable for use in the blood system owing to their special physicochemical properties and their higher stability, while organic nanoparticle platform-based anti-CTC nanomedicines are possibly more efficient to target the lymphatic system and have prolonged retention. It is expected that the integration of multifunctional inorganic nanoparticle(s) into organic nanoparticle platform or the organic coating/encapsulation of inorganic nanoparticle(s) will make nanomedicines more powerful and versatile in targeted theranostics of CTCs in the lymphatic system.234 Therefore, it is desirable to select and integrate suitable nanoparticle platforms for constructing high-efficacy anti-CTCs nanomedicines according to individualized requirements.
Figure 10.
Nanotheranostics of CTCs in the lymphatic circulation system with multifunctional dual-targeting nanomedicines. The dual-targeting nanomedicines can be engineered to target lymphatic nodes (LNs) and enter the lymphatic circulation system firstly, and then target the CTCs in the lymphatic circulation system. The dual-targeting will enable the diagnosis and responsive therapy of CTCs in the lymphatic circulation system.
6. Battling metastases (secondary) tumours by nanomedicines
After surviving in circulation systems, a very small number of CTCs tend to extravasate into the parenchyma of distant tissues (denoted as disseminated tumour cells, DTCs) and then form metastatic tumours. This process can be divided generally into three micro-steps: solitary cells, pre-angiogenesis micro-metastatic tumours, and vascularized macro-metastatic tumours. In principle, the interdiction of any micro-step in this progress can overcome tumoural metastases. Present alternative therapy routes include anti-proliferation and anti-growth of metastatic cells, anti-angiogenesis and anti-lymphangiogenesis.235 However once these DTCs survive in the foreign microenvironment of distant tissue, they will further evolve to become much more stubborn so that it is almost impossible to control their speedy proliferation and growth by present therapy routes. Therefore, it is better to battle the DTCs (termed “seeds”) by enhancing counteraction of the pre-metastatic niche (PMN, termed “soil”). The following Section will introduce and extend this anti-metastasis strategy based on nanomedicines.
6.1. Soil- and seed-targeted nanomedicine therapy
During the metastatic colonization of DTCs, a metastasis-favoured environment, termed PMN, is co-created by DTCs and stromal cells in the secondary site as well as exosome of tumour and stromal cells in the primary tumour, including chemoattractants, metalloproteinases, chemokines, cytokines, etc.236 According to the popular seed and soil theory of metastasis, the PMN and the DTCs are identified as the soil and seeds, respectively.237 The principal strategy for battling metastatic tumours is to destroy the soil and meanwhile kill seeds in the early stage of metastasis. As for destroying the soil, the reprogramming/re-education of stromal cells in the secondary site is clinically thought to be much better than the simple depletion of them since the microenvironment is capable of normalizing tumour cells.152 As demonstrated in Fig. 11, a variety of agents have been developed to destroy the soil, including EGFR and VEGFR inhibitors for anti-angiogenesis and anti-lymphangiogenesis (EGFR mAb, Bevacizumab (VEGF mAb)),238 CSF-1R/CCR2 antagonists (CCL2 mAb, CSF-1R inhibitor) and TNFα inhibitors (CD40 mAb) for repolarization and re-education of tumour-associated macrophages,115, 159, 239–241 immunomodulators (FoxP3 mAbcam, CD25 mAb (Treg cells), IL-12 mAb, IL-10 mAb (TAMs, MDSCs)) for anti-inflammation and activation of immune responses,153, 242–246 matrix metalloproteinase inhibitors (MMP-2 mAb, MMP-3 mAb, MMP-9 mAb) for damage to metastatic niche,247, 248 etc. However, effective wide-spectrum anti-metastatic drugs are rarely available and general anti-cancer chemotherapeutics are inefficient, or even cause resistance, owing to difference of hallmarks between primary tumours and metastatic tumours. Moreover, the lack of on-site and specific targeting of some therapeutics, such as most metalloproteinase inhibitors (MPIs) and anti-angiogenesis agents, has frequently led to severe toxic side effects, or even unexpected effects of advancing metastasis.248
Figure 11.
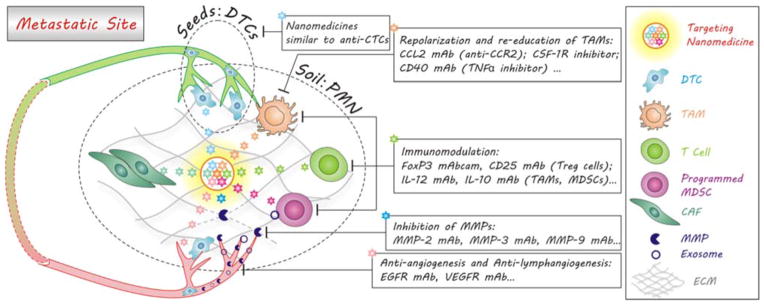
Soil- and seed-targeted delivery of nanomedicines for battling the pre-metastasis in the early stage of metastases. Besides targeting the seeds (DTCs) through optimized immuno-mediated recognition (Table 2), nanomedicines can be engineered to simultaneously target and destroy the soil (the pre-metastatic niche, PMN) by various routes including the repolarization of TAMs, the immunomodulation of stromal cells, the inhibition of MMPs, anti-angiogenesis and anti-lymphangiogenesis, where the synergistic therapy via the co-delivery of multi-drugs is applicable.
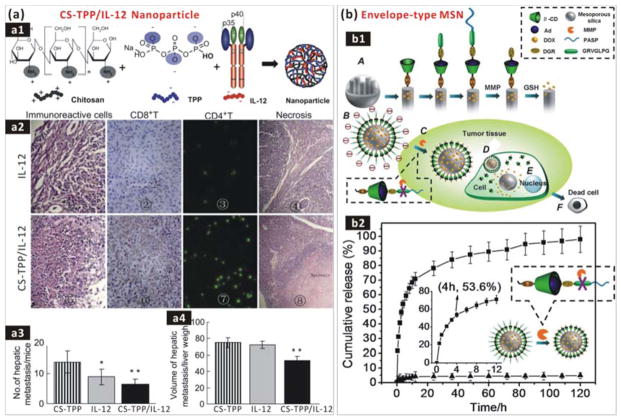
The high payload, soil-targeted delivery and responsive release of these agents in the pre-metastatic niche (the soil) by using nanomedicines is expected to be capable of more efficient damage to soil and seeds as well as avoidance/depression of toxic side effects of drugs which are derived from their non-specific inhibitions over the whole body (Fig. 11). Xu et al. encapsulated interluekin-12 (IL-12) into chitosan–tripolyphosphate (CS-TPP) cross-linked nanoparticles to build a CS-TPP/IL-12 nanoformula for liver metastasis-targeted drug delivery and immunotherapy (Fig. 12a1).249 Liver-localized CS-TPP/IL-12 nanomedicine was able to release IL-12 in a sustained and acid-responsive way, exhibiting a relatively low toxicity compared with free drug administration. Moreover, systemic delivery of the CS-TPP/IL-12 nanomedicine more efficiently induced the recruitment and tumour infiltration of natural killer T (NKT) cells, and consequently reduced the number and volume of colorectal cancer (CRC) liver metastasis foci compared to treatment with free IL-12. In addition, the IL-12 loading capacity of CS-TPP was considerably low (no more than 0.1 μg/mg) owing to the competition between TPP and IL-12 for electrostatic adsorption of chitosan (Fig. 12a1). A higher payload may be achievable using other advanced nano-carriers with high surface areas or/and hollow nano-structure, such as hMSNs, GO and Au nanocage, which will benefit the decrease of drug use amount and the enhancement of drug delivery and therapy efficacies.47, 250–252
Figure 12.
(a) Targeted delivery of CS-TPP/IL-12 nanomedicine: (a1) Construction of nanomedicine by electrostatic co-assembly of CS, TPP and IL-12; (a2) Evaluation of the metastasis immune response by Hematoxylin-Eosin immunohistochemical staining of hepatic metastasis immunoreactive cells in CRC hepatic metastasis model mice treated with free IL-12 and CS-TPP/IL-12 nanomedicine; (a3) The number and volume (a4) of hepatic metastasis in mice treated with CS-TPP carrier, IL-12 drug, CS-TPP/IL-12 nanomedicine.249 Reproduced with permission from ref. 249, Copyright 2012 Elsevier. (b) MMP-responsive drug delivery of the envelope-type MSN nanomedicine. (b1) Construction and responsive mechanism of nanomedicine: (A) functionalization protocol; (B) constructed nanomedicine; (C) undressing of PASP in response to MMP at a tumour site; (D) RGD-mediated uptake; (E) glutathione-triggered drug release inside the cell; (F) apoptosis of tumour cells. (b2) Undressing/release of the PASP protection layer in the presence of MMP-2 (■) or MMP-2 plus MMP inhibitor (▲).253 Reproduced with permission from ref. 253, Copyright 2013 American Chemical Society.
A range of receptors and antigens over-expressed on CTCs (the closest precursor of DTCs) and intratumoural vasculature are potential targets of seeds and soil, and thus corresponding targeting ligands (Table 2) can be used to conjugate nano-carriers for targeted drug delivery. Cancer-associated proteases (CAPs), which play important roles in the establishment of the soil, are drug therapeutic targets as well as targets for responsive drug delivery and release. For instance, Zhang et al. coated a MMP substrate peptide (Pro-Leu-Gly-Val-Arg, PLGVR) onto the surface of MSN carrier to realize MMP-responsive drug delivery (Fig. 12b1).253 After exposure to the MMP-rich PMN, MMP responsive hydrolysis of PLGVR led to undressing of the outermost protective layer (Fig. 12b2) and uncovering of RGD motif, which mediated the targeted delivery of nanomedicine into tumour tissue and cells (Fig. 12b1C). Then glutathione triggered the release of encapsulated drug inside tumour cells by cleaving disulfide chains (Fig. 12b1D). Moreover, a multistage nanoparticle delivery system was also constructed by using gelatin to encapsulate 10-nm nanoparticles into 100-nm ones in order to enhance the penetration of drugs into tumour tissue. The 100-nm delivery system was accumulated around the leaky regions of the tumour vasculature by the EPR effect, then degraded by CAPs such as MMP-2 in the tumour microenvironment, and subsequently released 10-nm nanoparticles for imaging and therapy. It was demonstrated that the MMP-2 activation of the multistage nanoparticles facilitated delivery into the dense collagen matrix of tumour.254
It should be noted that there are both overlapping and difference in anti-metastatic strategies for blocking primary and metastasis tumours (Sections 4 and 6). The overlap lies in similar anti-invasion/anti-intravasation and anti-angiogenesis methodologies. The two FDA-approved anti-metastatic drugs (angiogenesis and MMP inhibitors) can therefore play important roles separately or cooperatively in both primary and metastatic sites. However, the degrees of intratumoural vascularization in these two sites are somewhat different: primary tumour has a well-developed vasculature, while metastasized tumour has virtually no vasculature or just a developing vasculature. The immature vasculature might provide a good chance for anti-angiogenesis, while such metastasis tumours are so small that they cannot be readily detectable clinically by the present medical technologies. After a metastatic tumour grows up, it becomes extremely difficult to treat, and therefore it is necessary to realize the early diagnosis of metastasis.255 Some nano-probes with high-resolution imaging capability, such as SPIONs for enhanced MRI and perfluorocarbon nano-emulsions for enhanced US imaging, can provide a chance to improve the outcome of early metastasis diagnosis.256–260
6.2. Tumour-homing cells-mediated delivery of nanomedicines
Homing is a specific multistep process, which was first described in the lymphocyte re-circulation.261 In a typical homing process, lymphocytes preferentially migrate from blood into specific tissues and secondary lymphoid organs, and return to the blood via lymph vessels and the thoracic duct, which is mediated by the interaction between lymphocyte homing receptors on lymphocytes and vascular addressins on endotheliocytes.262 Likewise, tumour-homing is also a complex and multistep process in which many types of cells travel from distant locations to a tumour site. Similar to the metastatic cascade of tumour cells, homing cells may be activated, intravasate, travel through the circulation, extravasate, migrate and undergo phenotypic changes when reaching the tumour site finally.263 Based on the homing property of tumour-homing cells, cell therapy and metastatic diagnosis have been developed for anti-cancer, especially anti-metastasis, by using tumour-homing cells as vehicles to deliver therapeutic and imaging agents. Cell therapy strategies based on nanomedicine-loaded tumour-homing cells (termed “tumour-homing nanomedicines”) are potentially attractive as nanomedicines can be facilely engineered to be taken up by cells temporarily and also capable of controlled excretion from cells, as demonstrated by Fig. 13. Furthermore, tumour-homing cells have an inimitable inherent targetability to metastatic tumour sites compared with existing active and passive molecular targeting technologies.264 By combining the advantages of tumour-homing cells and nanomedicines, high anti-metastasis therapeutic outcomes are expected.
Figure 13.
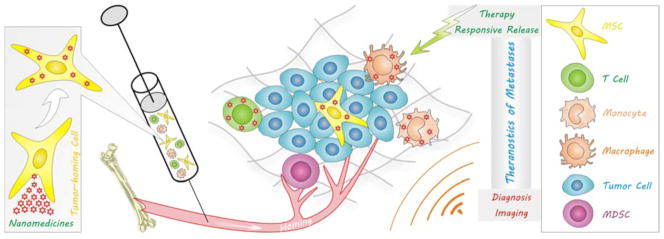
Tumour-homing cells-mediated delivery of nanomedicines to the metastatic site for theranostics of metastases. Nanomedicines can be loaded into or conjugated with tumour-homing cells, such as MSCs, T cells, monocytes and macrophages, and then delivered into metastases by the tumour-homing for diagnosis and therapy of metastasis.
Tumour-homing cells include mesenchymal stem cells (MSCs), monocytes (including derived tumour-associated macrophages), T cells, etc. These cells are homeostatically recruited from circulation systems into primary tumour and metastatic sites to establish tumour-favouring microenvironments, and can therefore be employed as vehicles of nanomedicine for targeted delivery (Fig. 13). Several typical examples of tumour-homing anti-metastasis cells-nanomedicines are introduced according to the type of cells as follows.
MSCs are well known to be inherently tumour-homing and immunosuppressive, and can also be isolated, cultured and expanded. These salient features render them especially suitable delivery vehicles for cell therapy of tumours. MSCs are capable of specifically and efficiently homing to primary and metastatic tumours from different administration routes. Loebinger et al. labelled MSCs with SPIONs to track MSCs and localize lung metastases by MRI.265 Their results indicated that i.v.-injected SPIONs-labelled MSCs could be tracked in vivo to multiple fresh lung metastases using MRI. NIR optical, MR, and PET multimodal imaging of metastases was realized by using MSNs as a versatile carrier platform to load Gd/64Cu/ZW800 and MSCs-mediated delivery of nanomedicines.266 It was also found that iron-based magnetic nanoparticles not only can label MSCs and track their fate by MRI, but also can enhance the tumour-homing capacity of MSCs by actively increasing the expression of chemokine receptor CXCR4 without the need for genetic modification.267 In addition, Kim et al. developed a kind of hollow MnO-coated MSNs to label MSCs through electroporation, and used them to successfully monitor the fate of transplanted MSCs in vivo over 14 days by MRI.268 Furthermore, Zhang et al. used pDNA-encapsulated polymer nanoparticles to transfect MSCs for expression of cytomegalovirus-thymidine kinase, which could induce a cell suicide effect by injection of ganciclovir. Almost all gene transfected MSCs were lodged in the lung area after systemic delivery and were also capable of migrating to metastatic nodules areas in the lung after 3 days. There the suicide effect of gene recombinant MSCs in the presence of ganciclovir caused sufficient bystander effect on lung metastases.269
Macrophages can be recruited into tumour sites and then educated to become tumour-associated macrophages (TAMs), which secrete intermediates to inhibit immunity and assist intravasation and extravasation of metastatic tumour cells, homing to metastatic sites. Therefore, macrophages may be ideal vehicles for targeting metastatic sites in principle.270 Ikehara et al. used macrophages to deliver 5-FU/SPIONs-loaded liposomes to metastatic tumour sites, and the SPIONs could play theranostic roles for MRI and hyperthermia therapy at the tumour sites, and effectively control the peritoneal metastasis.271 However, it was also found that majority of macrophages were entrapped by the reticuloendothelial systems (including lung, liver and spleen) prior to arriving metastatic sites, leading to a low homing efficiency.272 But their monocyte precursors are highly mobile and can home directly to metastatic sites, and might therefore be a better choice for cell therapy compared with macrophages.273 Choi et al. used monocytes to deliver gold nanoshells into tumour sites for photothermal therapy.274, 275 They demonstrated that gold nanoshells, especially silica-coated ones, were capable of phagocytosis by monocytes in vitro, and gold nanoshells-loaded monocytes homed and infiltrated tumour spheroids after in vivo injection. Specifically, the homed gold nanoshells mediated a localized photothermal therapy upon exposure of tumour spheroids to a NIR laser, owing to the high NIR-photothermal efficacy of gold nanoshells.
T cells were also explored as vehicles for delivery of vectors for anticancer cell therapy, especially immunotherapy owing to their ability to induce nanomedicines-activated anti-tumour immune response.276, 277 Several types of nanoparticles (Al2O3 NPs, Fe3O4@ZnO NPs, CNTs-polymer NPs and Lipid NPs) have been conjugated with antigens or loaded with cytokines to construct nanomedicines/nanovaccines, which efficiently presented tumour antigens or appropriate immune-stimulatory signals via cytokines to T-cells to elicit a potent anti-tumour immune response.278–282 These nanovaccines-immunized T-cells could provide long-lasting therapeutic effects against tumours and metastases. In the case of immunotherapy, T cells are not only cell vehicles of nanomedicines but also therapeutic cells activated by nanomedicines. In addition, Au nanoshells and nanoparticles were also developed for theranostics (NIR-photothermal therapy and imaging) of metastatic tumours by T cell-mediated delivery.283, 284 The efficiency of T cell-mediated delivery of nanomedicines to tumours increased considerably compared to nanomedicines alone. However, their biodistribution results demonstrated that a large amount of nanomedicines also accumulated on multiple other sites including bone, liver, spleen and lung. To enhance the homing efficiency of carrier cells, the external manipulation of these cells through nanomedicines is desirable. In this context, we used Fe3O4@SiO2 core/shell nanoparticles to label NK cells, monitored the movement of labelled cells by MRI, and manipulated them to target tumour sites by an external magnetic field.285 We found that the tumour-infiltrating rate of NK cells was enhanced by 17-fold through the Fe3O4@SiO2 nanoparticle loading and magnetic field guidance, and the killing activity of NK cells was still maintained after the loading of nanoparticles. This approach could inspire future efforts to enhance the tumour-targeting efficiency of nanomedicine-carried tumour-homing cells via the external manipulation of functionalized nanomedicines.
Here we highlight several potential advantages of using tumour-homing cells as vehicles for delivery of nanomedicines as compared with the direct delivery of nanomedicines: 1) unique inherent targetability to primary and metastatic tumour sites; 2) immunosuppression and escape from the capture of RES; 3) high tumour penetrability; 4) high and various payloads for theranostics. However, there are also several aspects that need to be paid attention to: 1) nanomedicines would have no cytotoxicity to tumour-homing nanomedicines-delivered cells, but can responsively show cytotoxicity after being delivered to tumour sites; 2) nanomedicines would not leak out of tumour-homing cells during homing; 3) nanomedicines would maintain or even enhance the migratory and homing ability of tumour-homing cells. In addition, there will be significant challenges in the translation of tumour-homing cells-based nanomedicine delivery strategies. Especially, the biodistribution, kinetics and biosafety of nanomedicines-loaded tumour-homing cells have been rarely investigated. As shown in Table 4, we summarized the reported anti-cancer nanomedicines that were delivered by a variety of tumour-homing cells. It can be found that there remain many blanks worthy of study, especially on engineering of tumour-homing cells and nanomedicines for high tumour-targeting efficiency and theranostic multifunction and organic integration of them. Moreover, many other excellent platforms of drug delivery carriers (graphene nanosheets, hMSNs, hMCNs, hMOSNs, multifunctional rattle-structured MSNs, etc.) and imaging agents (UCNPs, QDs, etc.) can be good candidates to construct multifunctional nanomedicines and integrate them with cell platforms for multimodal imaging and combination therapy.52, 53, 97, 98, 286, 287
Table 4.
Tumour-homing nanomedicines for cell-mediated therapy and imaging of primary tumours and metastases.
| Nanomedicines | Tumour-Homing Cells | ||
|---|---|---|---|
| MSCs | Macrophages | T Cells | |
| Therapy of Metastases | Spermine-Pullulan copolymer-pDNA for suicide gene therapy of pulmonary metastases269 | Liposomes-5FU/SPIONs for chemo-hyperthermal therapy of peritoneal metastasis271 | Au nanoshells for photothermal therapy of metastatic melanoma283 Al2O3 NPs-antigens for immunotherapy of metastatic cancers279 |
| Therapy of Primary Tumours | Silica nanorattle-DOX for chemotherapy of the U251 glioma tumour288 Hollow silica NPs-Pp18 for PDT therapy of breast tumours289 PMMA nanoparticles-TPPS for PDT therapy of osteosarcoma290 |
Au nanoshells for photothermal therapy of gliomas274, 275, 291, 292 | Fe3O4@ZnO NPs-antigens for cancer theranostics by immunotherapy and MRI280 CNTs@polymer-cytokines for immunotherapy of tumours281 Lipid NPs-cytokines for immunotherapy of lung and bone marrow tumours282 SPIONs for hyperthermal therapy of tumours293 BC NPs-peptides for Boron neutron capture therapy294 |
| Imaging of Metastases | SPIONs for MR imaging of lung metastases265 MSNs-Gd/64Cu/ZW800 for NIR optical, MR, PET multimodal imagings of the orthotopic U87MG glioblastoma xenograft and tumour draining SLNs and tumour metastasis diagnosis266 |
Ferromagnetic iron-oxide nanocubes (FIONs) for MR imaging of metastatic lymph nodes295 | – |
| Imaging of Primary Tumours | Au nanocages for photoacoustic imaging of glioblastoma296 SPION@AuNPs for theranostics of hepatocellular carcinoma by MRI and photothermal therapy297 |
– | SPIONs for MR imaging of tumours298, 299 |
7. Summary and perspectives
In conclusion, compared with free drug, nanomedicine has several distinguished features and advantages: 1) targeted delivery will enable the optimized distribution of drugs, elevated drug availability, and consequently reduced toxic side effects of drug; 2) the encapsulation of drugs favours the protection of its activity from biodegradation prior to reaching the tumour foci; 3) the enhancement of drug loading and delivery efficiencies can help minimize the dosage of drug; 4) sustained drug release corresponds to suppressed drug concentration fluctuation and decreased frequency of drug administration, while stimuli-responsive drug release ensures suppression of nonspecific leakage of drug in normal tissues and blood and thus reduce the toxic side effects of drug; 5) nanoparticles are capable of mediating transmembrane transport of encapsulated drug, and thus preventing drug excretion and favouring drug localized accumulation; 6) facile multifunction integration allows the combination of multimodal imaging, diagnosis and therapy; and 7) multifunctional nanoparticles possessing specific physicochemical properties can be developed for a range of therapy and imaging studies, such as PTT, PDT, HTT, PTI, AI, FI, MRI, PET, etc. The FDA office of pharmaceutical science announced that the trend in the development of nanomedicines is to make the nanoparticles multifunctional and controllable by external signals or local environments.300 Owing to facile multifunction integrability and a variety of unique physicochemical properties and theranostic functions, more nanomedicines based on inorganic nanoparticle platforms will be developed and play an significant role in anti-metastasis. In addition, the biological effect/safety of nanomedicine is also an important aspect that needs to be addressed, which involves biocompatibility (including toxicity, blood and tissue compatibility) and pharmacokinetics (including biodistribution, biodegradation, retention, excretion, clearance, blood circulation). Especially for some emerging nanomedicines based on inorganic nanomaterials, their biological effects are urgently needed to be investigated systematically but in a standardized and uniform way.
Aiming at the metastatic cascade, a variety of individualized anti-metastasis strategies by engineering nanomedicines are presently available (Fig. 14). A range of multifunctional nanoparticles are optional for constructing the desired nanomedicines, typically capable of the multi-targeted co-delivery of multi-drugs and imaging agents. These state-of-the-art nanomedicines will benefit many aspects of metastasis treatments: 1) high-resolution and accurate localization of lesions, early and less burdensome treatments, increased chances of recovery via cancer imaging and early diagnosis; 2) enhanced therapy efficacies, decreased drug dosage and administration frequency, and reduced toxic side effects on healthy tissues by targeted delivery and stimuli-responsive/localized/sustained release of largely loaded various therapeutics, intrinsic therapies of nanoparticles and combination of various therapy methods; 3) the combination of diagnosis and therapy.
Figure 14.
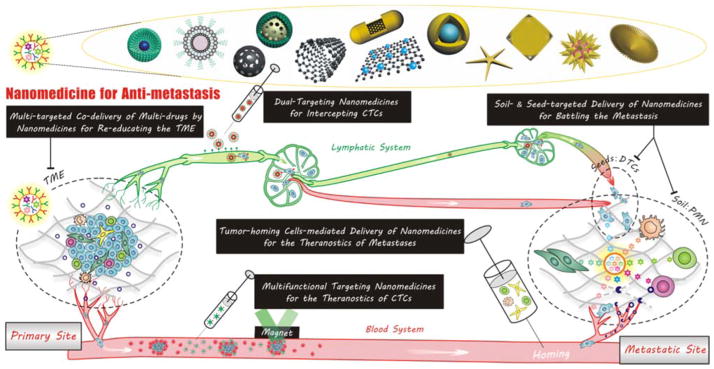
Summary of multifunctional anti-metastatic nanomedicines and nanomedicine-based anti-metastasis strategies aiming at each stage of the metastatic cascade.
Extraordinarily strong resistance of metastases to treatments accounts for failure of various anti-metastasis therapies. The resistance of cancer follows the evolutionary and biological logic, where the resistance of metastasis to treatments ever-increasingly strengthens with the progression of metastasis as well as the intervention of therapies. As the immunity system of human body is already considerably powerful, the vitality and resistivity of metastasis should be much stronger. Only relying on existing routine therapy strategies, even relatively efficient radiochemotherapy and surgical excision, the treatment of metastasis will not succeed. In this context, immunotherapy is extensively thought as the optimal choice for treatment of metastasis at present because its mechanism is to stimulate/strengthen the immunity of human body or/and selectively disable the immunosuppression of the tumour microenvironment for thoroughly killing rare tumour metastatic cells by immune cells, rather than simply and directly struggling against metastatic tumour cells like most of other therapies against primary tumours. Therefore, immunotherapy will result in much lower toxicity on normal tissues and suppressed resistance of metastasis. However currently, a main problem of immunotherapy is the non-specific immunotoxicity owing to the off-target effect. Therefore, targeted nanomedicines will play an important role in reducing the immunotoxicity of immunotherapy. In addition, with the assistance of other therapies, such as chemotherapy, radiotherapy, PDT and thermotherapy, the anti-metastasis efficacy of immunotherapy will be amplified by attacking tumour metastatic cells from multiple angles.
Based on the same evolutionary theory of cancer, human body exhibits a considerably strong immune defence mechanism to eliminate more than 99.99% of tumour cells. Therefore it is wise to further enhance the immunity function of human body for blocking the remaining 0.01% of CTCs from seeding in the soil (the pre-metastatic niche). Prior to seed germination, there is a better chance for nanomedicine-based therapy to block the completion of metastasis, indicating the significance of therapy of CTCs.
In summary, the metastatic cascade leaves us many opportunities to intervene, where advanced nanomedicines will play an important role. At the same time, anti-metastasis is also considerably challenging but significant. With the rapid development of nanotechnology and the emergence of various nanomaterials, more advanced and effective anti-metastatic nanomedicines are expectable to be engineered in the future.
Supplementary Material
Acknowledgments
We thank the financial support from the National Nature Science Foundation of China (Grant Nos. 51102259, 31271021 and 31222023), Distinguished Young Scholars of Sichuan University (Grant No. 2011SCU04B18) and the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Biographies

Qianjun He received his BSc and MSc respectively in Polymer and Inorganic Material Sciences at the Wuhan Institute of Technology, and obtained his PhD (2010) and then became an Assistant Professor at the Shanghai Institute of Ceramics, Chinese Academy of Sciences. In 2012 he was appointed as a Marie Curie Fellow at University of Leeds, UK. In 2014, he joined the Laboratory of Molecular Imaging and Nanomedicine, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, USA as a postdoc. His research focuses on the engineering of multifunctional nanomaterials for biomedical applications, especially in anti-cancer and gas therapy.

Shengrong Guo received his PhD in polymer physics & chemistry from Zhejiang University in 1995. He joined Fudan University as a Lecturer of Pharmaceutics in 1996 and was promoted to Associate Professor in 1999. He then moved to Shanghai JiaoTong University in 2000, and was promoted to Professor, Principal Investigator and Director of the Group of Biomedical Polymer and Drug Controlled Release in 2003. He has published over 100 papers and 4 books. His group focuses on novel drug delivery systems for cancer treatment, including nanomedicines and medical device/drug combinations.

Zhiyong Qian obtained his PhD from Chengdu Institute of Organic Chemistry CAS in 2003 under the supervision of Prof. Xiaobo Liu, and then carried out his postdoctoral work at Sichuan University under the supervision of Prof. Mingjing Tu (Academician of Chinese Academy of Engineering). In 2005 he was promoted to Full Professor of State Key Lab of Biotherapy, and nominated as a Leading Scientist of Sichuan University for the National 985 Project of China. His research interests are in the area of biomaterials, biomedical engineering, drug delivery system and nanotechnology. He has published more than 90 papers in peer-reviewed journals.

Xiaoyuan Chen received his PhD in chemistry from the University of Idaho in 1999. After being a faculty member at the University of Southern California and Stanford University. He joined the Intramural Research Program of the NIBIB in 2009 as a Senior Investigator and Chief of the Laboratory of Molecular Imaging and Nanomedicine (LOMIN). Dr Chen has published over 450 papers and numerous books and book chapters. He sits on the editorial board of over 10 peer-reviewed journals and is the founding editor of the journal Theranostics. His lab focuses on developing molecular imaging probes and nanotechnologies for early diagnosis of disease, monitoring therapy responses, and guiding drug discovery/development.
Notes and references
- 1.Lu DY, Lu TR, Wu HY. Adv Tech Biol Med. 2013;1:1000106. [Google Scholar]
- 2.Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang JS, Hwang YK, Marsaud V, Bories PN, Cynober L, Gil S, Ferey G, Couvreur P, Gref R. Nat Mater. 2010;9:172–178. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Nat Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallet-Regi M, Ramila A, del Real RP, Perez-Pariente J. Chem Mater. 2001;13:308–311. [Google Scholar]
- 5.Nandiyanto ABD, Kim SG, Iskandar F, Okuyama K. Microporous Mesoporous Mater. 2009;120:447–453. [Google Scholar]
- 6.Polshettiwar V, Cha D, Zhang XX, Basset JM. Angew Chem Int Edit. 2010;49:9652–9656. doi: 10.1002/anie.201003451. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YF, Shi JL, Shen WH, Chen HR, Dong XP, Ruan ML. Nanotechnol. 2005;16:2633–2638. [Google Scholar]
- 8.You J, Zhang GD, Li C. ACS Nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon GD, Choi SW, Cai X, Li WY, Cho EC, Jeong U, Wang LV, Xia YN. J Am Chem Soc. 2011;133:4762–4765. doi: 10.1021/ja200894u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan WP, Shen B, Bu WB, Chen F, Zhao KL, Zhang SJ, Zhou LP, Peng WJ, Xiao QF, Xing HY, Liu JN, Ni DL, He QJ, Shi JL. J Am Chem Soc. 2013;135:6494–6503. doi: 10.1021/ja312225b. [DOI] [PubMed] [Google Scholar]
- 11.Wu HX, Zhang SJ, Zhang JM, Liu G, Shi JL, Zhang LX, Cui XZ, Ruan ML, He QJ, Bu WB. Adv Funct Mater. 2011;21:1850–1862. [Google Scholar]
- 12.Chen Y, Chen HR, Zhang SJ, Chen F, Sun SK, He QJ, Ma M, Wang X, Wu HX, Zhang LX, Zhang LL, Shi JL. Biomaterials. 2012;33:2388–2398. doi: 10.1016/j.biomaterials.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 13.Zhou ZX, Ma XP, Murphy CJ, Jin EL, Sun QH, Shen YQ, Van Kirk EA, Murdoch WJ. Angew Chem Int Edit. 2014;53:10949–10955. doi: 10.1002/anie.201406442. [DOI] [PubMed] [Google Scholar]
- 14.Kojima C, Suehiro T, Watanabe K, Ogawa M, Fukuhara A, Nishisaka E, Harada A, Kono K, Inui T, Magata Y. Acta Biomaterialia. 2013;9:5673–5680. doi: 10.1016/j.actbio.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Zhu SJ, Hong MH, Tang GT, Qian LL, Lin JY, Jiang YY, Pei YY. Biomaterials. 2010;31:1360–1371. doi: 10.1016/j.biomaterials.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Shen YQ, Zhou ZX, Sui MH, Tang JB, Xu PS, Van Kirk EA, Murdoch WJ, Fan MH, Radosz M. Nanomedicine. 2010;5:1205–1217. doi: 10.2217/nnm.10.86. [DOI] [PubMed] [Google Scholar]
- 17.Navath RS, Kurtoglu YE, Wang B, Kannan S, Romero R, Kannan RM. Bioconjugate Chem. 2008;19:2446–2455. doi: 10.1021/bc800342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JMJ, Dy EE, Szoka FC. Proc Natl Acad Sci U S A. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminskas LM, McLeod VM, Ryan GM, Kelly BD, Haynes JM, Williamson M, Thienthong N, Owen DJ, Porter CJH. J Control Release. 2014;183:18–26. doi: 10.1016/j.jconrel.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Parrott MC, Benhabbour SR, Saab C, Lemon JA, Parker S, Valliant JF, Adronov A. J Am Chem Soc. 2009;131:2906–2916. doi: 10.1021/ja8078175. [DOI] [PubMed] [Google Scholar]
- 21.Talanov VS, Regino CAS, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Nano Lett. 2006;6:1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Shirakawa K, Kawamoto S, Saga T, Sato N, Hiraga A, Watanabe I, Heike Y, Togashi K, Konishi J, Brechbiel MW, Wakasugi H. Cancer Res. 2002;62:860–866. [PubMed] [Google Scholar]
- 23.Xie JJ, Zhao RL, Gu SG, Dong HY, Wang JC, Lu YS, Sinko PJ, Yu T, Xie FW, Wang L, Shao JW, Jia L. Theranostics. 2014;4:1250–1263. doi: 10.7150/thno.8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie JJ, Lu YS, Dong HY, Zhao RL, Chen HN, Shen WY, Sinko PJ, Zhu YW, Wang JC, Shao JW, Gao Y, Xie FW, Jia L. Adv Funct Mater. 2015;25:1304–1313. [Google Scholar]
- 25.Kobayashi H, Brechbiel MW. Adv Drug Deliver Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Kaminskas LM, Kelly BD, McLeod VM, Sberna G, Owen DJ, Boyd BJ, Porter CJH. J Control Release. 2011;152:241–248. doi: 10.1016/j.jconrel.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Kweon S, Lee HJ, Hyung WJ, Suh J, Lim JS, Lim SJ. Pharm Res. 2010;27:1408–1415. doi: 10.1007/s11095-010-0135-5. [DOI] [PubMed] [Google Scholar]
- 28.Chan S, Davidson N, Juozaityte E, Erdkamp F, Pluzanska A, Azarnia N, Lee LW, Grp MS. Ann Oncol. 2004;15:1527–1534. doi: 10.1093/annonc/mdh393. [DOI] [PubMed] [Google Scholar]
- 29.Cevc G, Schätzlein A, Gebauer D, Blume G. In: Prediction of Percutaneous Penetration. Bain KR, Hadgraft J, James WJ, Water KA, editors. STS Publishing; Cardiff, UK: 1993. pp. 226–234. [Google Scholar]
- 30.Woodle MC, Lasic DD. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZH, Yu Y, Dai WB, Lu JK, Cui JR, Wu HN, Yuan L, Zhang H, Wang XQ, Wang JC, Zhang X, Zhan Q. Biomaterials. 2012;33:8451–8460. doi: 10.1016/j.biomaterials.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Qin C, He B, Dai WB, Zhang H, Wang XQ, Wang JC, Zhang X, Wang GJ, Yin LF, Zhang Q. Mol Pharm. 2014;11:3233–3241. doi: 10.1021/mp400691z. [DOI] [PubMed] [Google Scholar]
- 33.Wang FF, Chen L, Zhang R, Chen ZP, Zhu L. J Control Release. 2014;196:222–233. doi: 10.1016/j.jconrel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Opanasopit P, Sakai M, Nishikawa M, Kawakami S, Yamashita F, Hashida M. J Control Release. 2002;80:283–294. doi: 10.1016/s0168-3659(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 35.Lukyanov AN, Torchilin VP. Adv Drug Deliver Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Xu PF, Yu HJ, Zhang ZW, Meng QS, Sun HP, Chen XZ, Yin Q, Li YP. Biomaterials. 2014;35:7574–7587. doi: 10.1016/j.biomaterials.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Sun L, Wu QJ, Guo WH, Li L, Chen YS, Li YC, Gong CY, Qian ZY, Wei YQ. Int J Pharm. 2013;443:175–182. doi: 10.1016/j.ijpharm.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Shen JA, Sun HP, Xu PF, Yin Q, Zhang ZW, Wang SL, Yu HJ, Li YP. Biomaterials. 2013;34:1581–1590. doi: 10.1016/j.biomaterials.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 39.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang SF, Moschetta M, Seevaratnam D, Zhang Y, Liu JH, Memarzadeh M, Wu J, Manier S, Shi JJ, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, Roccaro AM, Farokhzad OC, Ghobrial IM. Proc Natl Acad Sci U S A. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Biomaterials. 2012;33:7164–7173. doi: 10.1016/j.biomaterials.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Dreaden EC, Alkilany AM, Huang XH, Murphy CJ, El-Sayed MA. Chem Soc Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu CX, Niestroj M, Yuan D, Chang S, Chen J. Int J Nanomedicine. 2015;10:2065–2077. doi: 10.2147/IJN.S72144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lum CT, Liu X, Sun RWY, Li XP, Peng Y, He ML, Kung HF, Che CM, Lin MCM. Cancer Lett. 2010;294:159–166. doi: 10.1016/j.canlet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 44.Zhang WQ, Meng J, Ji YL, Li XJ, Kong H, Wu XC, Xu HY. Nanoscale. 2011;3:3923–3932. doi: 10.1039/c1nr10573f. [DOI] [PubMed] [Google Scholar]
- 45.Wang DG, Xu ZA, Yu HJ, Chen XZ, Feng B, Cui ZR, Lin B, Yin Q, Zhang ZW, Chen CY, Wang J, Zhang W, Li YP. Biomaterials. 2014;35:8374–8384. doi: 10.1016/j.biomaterials.2014.05.094. [DOI] [PubMed] [Google Scholar]
- 46.Lal S, Clare SE, Halas NJ. Acc Chem Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 47.Xia YN, Li WY, Cobley CM, Chen JY, Xia XH, Zhang Q, Yang MX, Cho EC, Brown PK. Acc Chem Res. 2011;44:914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang P, Rong PF, Lin J, Li WW, Yan XF, Zhang MG, Nie LM, Niu G, Lu J, Wang W, Chen XY. J Am Chem Soc. 2014;136:8307–8313. doi: 10.1021/ja503115n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson JT, Tabakman SM, Liang YY, Wang HL, Casalongue HS, Vinh D, Dai HJ. J Am Chem Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 50.Robinson JT, Welsher K, Tabakman SM, Sherlock SP, Wang HL, Luong R, Dai HJ. Nano Res. 2010;3:779–793. doi: 10.1007/s12274-010-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang K, Zhang SA, Zhang GX, Sun XM, Lee ST, Liu ZA. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Xu PF, Wu MY, Meng QS, Chen HR, Shu Z, Wang J, Zhang LX, Li YP, Shi JL. Adv Mater. 2014;26:4294–4301. doi: 10.1002/adma.201400303. [DOI] [PubMed] [Google Scholar]
- 53.Gu JL, Su SS, Li YS, He QJ, Shi JL. Chem Commun. 2011;47:2101–2103. doi: 10.1039/c0cc04598e. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Fan AC, Rakhra K, Sherlock S, Goodwin A, Chen XY, Yang QW, Felsher DW, Dai HJ. Angew Chem Int Edit. 2009;48:7668–7672. doi: 10.1002/anie.200902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel MA, Yang H, Chiu PL, Mastrogiovanni DD, Flach CR, Savaram K, Gomez L, Hemnarine A, Mendelsohn R, Garfunkel E, Jiang H, He H. ACS Nano. 2013;7:8147–8157. doi: 10.1021/nn403429v. [DOI] [PubMed] [Google Scholar]
- 56.Cao L, Wang X, Meziani MJ, Lu FS, Wang HF, Luo PJG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY, Sun YP. J Am Chem Soc. 2007;129:11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang YXJ, Hussain SM, Krestin GP. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 58.Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, Kim JG, Kim IS, Park KI, Cheon J. Nat Nanotechnol. 2011;6:418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- 59.Johannsen M, Gneueckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, Scholz R, Jordan A, Loening SA, Wust P. Eur Urol. 2007;52:1653–1662. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Chertok B, Moffat BA, David AE, Yu FQ, Bergemann C, Ross BD, Yang VC. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Nat Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 62.Birkhauser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A, Thoeny HC. Eur Urol. 2013;64:953–960. doi: 10.1016/j.eururo.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Z, Zhou Z, Bao J, Wang Z, Hu J, Chi X, Ni K, Wang R, Chen X, Chen Z, Gao J. Nat Commun. 2013;4:2266. doi: 10.1038/ncomms3266. [DOI] [PubMed] [Google Scholar]
- 64.Zhou ZJ, Huang DT, Bao JF, Chen QL, Liu G, Chen Z, Chen XY, Gao JH. Adv Mater. 2012;24:6223–6228. doi: 10.1002/adma.201203169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou ZJ, Wang LR, Chi XQ, Bao JF, Yang LJ, Zhao WX, Chen Z, Wang XM, Chen XY, Gao JH. ACS Nano. 2013;7:3287–3296. doi: 10.1021/nn305991e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng J, Qi T, Liao J, Chu B, Yang Q, Qu Y, Li W, Li H, Luo F, Qian Z. Theranostics. 2014;4:678–692. doi: 10.7150/thno.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma M, Chen H, Chen Y, Wang X, Chen F, Cui X, Shi J. Biomaterials. 2012;33:989–998. doi: 10.1016/j.biomaterials.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 68.Zhao WR, Gu JL, Zhang LX, Chen HR, Shi JL. J Am Chem Soc. 2005;127:8916–8917. doi: 10.1021/ja051113r. [DOI] [PubMed] [Google Scholar]
- 69.Grit M, Crommelin JA. Chem Phys Lipids. 1993;64:3–18. doi: 10.1016/0009-3084(93)90053-6. [DOI] [PubMed] [Google Scholar]
- 70.Mo R, Jiang TY, Gu Z. Nanomedicine. 2014;9:1117–1120. doi: 10.2217/nnm.14.62. [DOI] [PubMed] [Google Scholar]
- 71.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 72.Wu GH, Milkhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. J Am Chem Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang SL. Adv Drug Deliver Rev. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Deng C, Jiang YJ, Cheng R, Meng FH, Zhong ZY. Nano Today. 2012;7:467–480. [Google Scholar]
- 75.Kim S, Shi YZ, Kim JY, Park K, Cheng JX. Expert Opin Drug Del. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 76.Wang CG, Irudayaraj J. Small. 2010;6:283–289. doi: 10.1002/smll.200901596. [DOI] [PubMed] [Google Scholar]
- 77.Elliott AM, Shetty AM, Wang J, Hazle JD, Stafford RJ. Int J Hyperthermia. 2010;26:434–440. doi: 10.3109/02656731003685805. [DOI] [PubMed] [Google Scholar]
- 78.Yavuz MS, Cheng YY, Chen JY, Cobley CM, Zhang Q, Rycenga M, Xie JW, Kim C, Song KH, Schwartz AG, Wang LHV, Xia YN. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vivero-Escoto JL, Slowing II, Wu CW, Lin VSY. J Am Chem Soc. 2009;131:3462–3463. doi: 10.1021/ja900025f. [DOI] [PubMed] [Google Scholar]
- 80.Tong L, Wei QS, Wei A, Cheng JX. Photochem Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang HW, Liu HL, Li ML, Hsi IW, Fan CT, Huang CY, Lu YJ, Hua MY, Chou HY, Liaw JW, Ma CCM, Wei KC. Biomaterials. 2013;34:5651–5660. doi: 10.1016/j.biomaterials.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 82.Deng H, Zhong YQ, Du MH, Liu QJ, Fan ZM, Dai FY, Zhang X. Theranostics. 2014;4:904–918. doi: 10.7150/thno.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang HF, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng JX. Proc Natl Acad Sci U S A. 2005;102:15752–15756. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cang H, Sun T, Li ZY, Chen JY, Wiley BJ, Xia YN, Li XD. Opt Lett. 2005;30:3048–3050. doi: 10.1364/ol.30.003048. [DOI] [PubMed] [Google Scholar]
- 85.He QJ, Shi JL. J Mater Chem. 2011;21:5845–5855. [Google Scholar]
- 86.Chen Y, Chen HR, Guo LM, He QJ, Chen F, Zhou J, Feng JW, Shi JL. ACS Nano. 2010;4:529–539. doi: 10.1021/nn901398j. [DOI] [PubMed] [Google Scholar]
- 87.He QJ, Zhang ZW, Gao F, Li YP, Shi JL. Small. 2011;7:271–280. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 88.Fu CH, Liu TL, Li LL, Liu HY, Chen D, Tang FQ. Biomaterials. 2013;34:2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 89.Fan W, Shen B, Bu W, Chen F, He Q, Zhao K, Zhang S, Zhou L, Peng W, Xiao Q, Ni D, Liu J, Shi J. Biomaterials. 2014;35:8992–9002. doi: 10.1016/j.biomaterials.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. J Am Chem Soc. 2003;125:4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 91.Yang PP, Gai SL, Lin J. Chem Soc Rev. 2012;41:3679–3698. doi: 10.1039/c2cs15308d. [DOI] [PubMed] [Google Scholar]
- 92.Wang X, Chen HR, Chen Y, Ma M, Zhang K, Li FQ, Zheng YY, Zeng DP, Wang Q, Shi JL. Adv Mater. 2012;24:785–791. doi: 10.1002/adma.201104033. [DOI] [PubMed] [Google Scholar]
- 93.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Angew Chem Int Edit. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 94.Lee CH, Cheng SH, Wang YJ, Chen YC, Chen NT, Souris J, Chen CT, Mou CY, Yang CS, Lo LW. Adv Funct Mater. 2009;19:215–222. [Google Scholar]
- 95.Yang K, Hu LL, Ma XX, Ye SQ, Cheng L, Shi XZ, Li CH, Li YG, Liu Z. Adv Mater. 2012;24:1868–1872. doi: 10.1002/adma.201104964. [DOI] [PubMed] [Google Scholar]
- 96.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Nat Nanotechnol. 2009;4:688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He Q, Shi J. Adv Mater. 2014;26:391–411. doi: 10.1002/adma.201303123. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Meng QS, Wu MY, Wang SG, Xu PF, Chen HR, Li YP, Zhang LX, Wang LZ, Shi JL. J Am Chem Soc. 2014;136:16326–16334. doi: 10.1021/ja508721y. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y, Xu PF, Chen HR, Li YS, Bu WB, Shu Z, Li YP, Zhang JM, Zhang LX, Pan LM, Cui XZ, Hua ZL, Wang J, Zhang LL, Shi JL. Adv Mater. 2013;25:3100–3105. doi: 10.1002/adma.201204685. [DOI] [PubMed] [Google Scholar]
- 100.Chen YJ, Gu HC, Zhang DSZ, Li F, Liu TY, Xia WL. Biomaterials. 2014;35:10058–10069. doi: 10.1016/j.biomaterials.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U. Nano Lett. 2005;5:113–117. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]
- 102.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye YP, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Sci Transl Med. 2014:6. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosenholm JM, Mamaeva V, Sahlgren C, Linden M. Nanomedicine. 2012;7:111–120. doi: 10.2217/nnm.11.166. [DOI] [PubMed] [Google Scholar]
- 104.Wang S, Zhang Q, Luo XF, Li J, He H, Yang F, Di Y, Jin C, Jiang XG, Shen S, Fu DL. Biomaterials. 2014;35:9473–9483. doi: 10.1016/j.biomaterials.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 105.Yang F, Jin C, Yang D, Jiang YJ, Li J, Di Y, Hu JH, Wang CC, Ni QX, Fu DL. Eur J Cancer. 2011;47:1873–1882. doi: 10.1016/j.ejca.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 106.Kong QL, Zhang LX, Liu JN, Wu MY, Chen Y, Feng JW, Shi JL. Chem Commun. 2014;50:15772–15775. doi: 10.1039/c4cc07121b. [DOI] [PubMed] [Google Scholar]
- 107.Khandare J, Calderon M, Dagia NM, Haag R. Chem Soc Rev. 2012;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- 108.Ardana A, Whittaker AK, Thurecht KJ. Macromolecules. 2014;47:5211–5219. [Google Scholar]
- 109.Coles DJ, Rolfe BE, Boase NRB, Veedu RN, Thurecht KJ. Chem Commun. 2013;49:3836–3838. doi: 10.1039/c3cc00127j. [DOI] [PubMed] [Google Scholar]
- 110.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu JW, Phillips B, Carter MB, Carroll NJ, Jiang XM, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu JW, Stace-Naughton A, Jiang XM, Brinker CJ. J Am Chem Soc. 2009;131:1354–1355. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vivero-Escoto JL, Rieter WJ, Lau H, Huxford-Phillips RC, Lin WB. Small. 2013;9:3523–3531. doi: 10.1002/smll.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valastyan S, Weinberg RA. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang JA, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Veiseh O, Kievit FM, Ellenbogen RG, Zhang M. Adv Drug Deliv Rev. 2011;63:582–596. doi: 10.1016/j.addr.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie J, Chen K, Lee HY, Xu CJ, Hsu AR, Peng S, Chen XY, Sun SH. J Am Chem Soc. 2008;130:7542–7543. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Proc Natl Acad Sci U S A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu ZF, Wang F, Chen XY. Drug Dev Res. 2008;69:329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen XY. Recent Pat Anticancer Drug Discov. 2007;2:143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- 122.Veeravagu A, Hsu AR, Cai WB, Hou LC, Tse VCK, Chen XY. Recent Pat Anticancer Drug Discov. 2007;2:59–71. doi: 10.2174/157489207779561426. [DOI] [PubMed] [Google Scholar]
- 123.Cai W, Chen X. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 124.Dayam R, Aiello F, Deng JX, Wu Y, Garofalo A, Chen XY, Neamati N. J Med Chem. 2006;49:4526–4534. doi: 10.1021/jm051296s. [DOI] [PubMed] [Google Scholar]
- 125.Zhen ZP, Tang W, Chen HM, Lin X, Todd T, Wang G, Cowger T, Chen XY, Xie J. ACS Nano. 2013;7:4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang F, Huang XL, Zhu L, Guo N, Niu G, Swierczewska M, Lee S, Xu H, Wang AY, Mohamedali KA, Rosenblum MG, Lu GM, Chen XY. Biomaterials. 2012;33:5414–5422. doi: 10.1016/j.biomaterials.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai WB, Chen K, Li ZB, Gambhir SS, Chen XY. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 128.Bhirde A, Guo N, Chen XY. Theranostics. 2011;1:274–276. doi: 10.7150/thno/v01p0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen K, Xie J, Chen XY. Mol Imaging. 2009;8:65–73. [PMC free article] [PubMed] [Google Scholar]
- 130.Cai WB, Chen XY. Front Biosci. 2007;12:4267–4279. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- 131.Lee HY, Li Z, Chen K, Hsu AR, Xu CJ, Xie J, Sun SH, Chen XY. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 132.Niu G, Xiong ZM, Cheng Z, Cai WB, Gambhir SS, Xing L, Chen XY. Mol Imaging Biol. 2007;9:126–134. doi: 10.1007/s11307-007-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Z, Huang P, Bhirde A, Jin A, Ma Y, Niu G, Neamati N, Chen XY. Chem Commun. 2012;48:12261–12261. doi: 10.1039/c2cc31974h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JSH, Zhang MQ. Small. 2009;5:256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, Zhang MQ. ACS Nano. 2010;4:4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, Mok H, Ellenbogen RG, Park JO, Zhang MQ. Biomaterials. 2010;31:8032–8042. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang F, Xie J, Liu G, He YL, Lu GM, Chen XY. Mol Imaging Biol. 2011;13:695–701. doi: 10.1007/s11307-010-0401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eckert MA, Yang J. Oncotarget. 2011;2:562–568. doi: 10.18632/oncotarget.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paz H, Pathak N, Yang J. Oncogene. 2014;33:4193–4202. doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, Postenka CO, Turley EA, Courtneidge SA, Chambers AF, Lewis JD. Cell Rep. 2014;8:1558–1570. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi H. Eur J Cell Biol. 2012;91:902–907. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 142.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, Leslie MC, Vivas-Mejia PE, Lopez-Berestein G, Sood AK, Bar-Eli M. Cancer Res. 2008;68:9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gonda K, Watanabe TM, Ohuchi N, Higuchi H. J Biol Chem. 2010;285:2750–2757. doi: 10.1074/jbc.M109.075374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pan LM, Liu JN, He QJ, Shi JL. Adv Mater. 2014;26:6742–6748. doi: 10.1002/adma.201402752. [DOI] [PubMed] [Google Scholar]
- 145.Kang SG, Zhou GQ, Yang P, Liu Y, Sun BY, Huynh T, Meng H, Zhao LN, Xing GM, Chen CY, Zhao YL, Zhou RH. Proc Natl Acad Sci U S A. 2012;109:15431–15436. doi: 10.1073/pnas.1204600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meng H, Xing GM, Blanco E, Song Y, Zhao LN, Sun BY, Li XD, Wang PC, Korotcov A, Li W, Liang XJ, Chen CY, Yuan H, Zhao F, Chen Z, Sun T, Chai ZF, Ferrari M, Zhao YL. Nanomed-Nanotechnol. 2012;8:136–146. doi: 10.1016/j.nano.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alili L, Sack M, von Montfort C, Giri S, Das S, Carroll KS, Zanger K, Seal S, Brenneisen P. Antioxid Redox Sign. 2013;19:765–778. doi: 10.1089/ars.2012.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S, Seal S, Brenneisen P. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 149.Lucafo M, Pelillo C, Carini M, Da Ros T, Prato M, Sava G. Nano-Micro Lett. 2014;6:163–168. [Google Scholar]
- 150.Wang ZX, Hou Y, Han W, Wang KC, Guo BF, Liu Y, Chang XH, Wang WH, Na WL, Kong XB, Zhao X, Zhang L. Chem Res Chin Univ. 2011;27:94–98. [Google Scholar]
- 151.Liu ZX, Wu YC, Guo ZR, Liu Y, Shen YJ, Zhou P, Lu X. PLoS One. 2014:9. doi: 10.1371/journal.pone.0099175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Quail DF, Joyce JA. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Nature. 2007;449:557–U554. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 155.Hanahan D, Weinberg RA. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 156.Albini A, Sporn MB. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 157.O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R. Proc Natl Acad Sci U S A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD, Venkata KCN, Rosenberg DO, Petasis NA, Lenz HJ, Hong YK. Brit J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun WJ, Huhn RD, Song WR, Li DG, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma P, Wagner K, Wolchok JD, Allison JP. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen DS, Irving BA, Hodi FS. Clin Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 162.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen XD, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 163.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao YY, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gubin MM, Zhang XL, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SSK, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJM, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mellman I, Coukos G, Dranoff G. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jain RK. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chauhan VP, Jain RK. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stylianopoulos T, Jain RK. Proc Natl Acad Sci U S A. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han XX, Adstamongkonkul P, Popovic Z, Huang PG, Bawendi MG, Boucher Y, Jain RK. Nat Commun. 2013;4 doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Datta M, Via LE, Kamoun WS, Liu C, Chen W, Seano G, Weiner DM, Schimel D, England K, Martin JD, Gao X, Xu L, Barry CE, Jain RK. Proc Natl Acad Sci U S A. 2015;112:1827–1832. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang YH, Yuan JP, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kashiwagi S, Tsukada K, Xu L, Miyazaki J, Kozin SV, Tyrrell JA, Sessa WC, Gerweck LE, Jain RK, Fukumura D. Nat Med. 2008;14:255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- 175.Jain RK. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang XW, Tian WX, Cai XZ, Wang XF, Dang WQ, Tang H, Cao H, Wang L, Chen TM. PLoS One. 2013:8. doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H, Manabe T. Mol Cancer. 2006;5 doi: 10.1186/1476-4598-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qian BZ, Li JF, Zhang H, Kitamura T, Zhang JH, Campion LR, Kaiser EA, Snyder LA, Pollard JW. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, Masui T, Fujimoto K, Tamamura H, Hiramatsu K, Fujii N, Imamura M. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 180.Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen XY. J Nucl Med. 2010;51:1796–1804. doi: 10.2967/jnumed.110.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jacobson O, Weiss ID, Szajek LP, Niu G, Ma Y, Kiesewetter DO, Farber JM, Chen XY. Theranostics. 2011;1:251–262. doi: 10.7150/thno/v01p0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weiss ID, Jacobson O, Kiesewetter DO, Jacobus JP, Szajek LP, Chen XY, Farber JM. Mol Imaging Biol. 2012;14:106–114. doi: 10.1007/s11307-010-0466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang XX, Sun ZC, Guo JX, Wang Z, Wu CX, Niu G, Ma Y, Kiesewetter DO, Chen XY. Mol Imaging Biol. 2013;15:758–767. doi: 10.1007/s11307-013-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.He YL, Song W, Lei J, Li Z, Cao J, Huang S, Meng J, Xu HY, Jin ZY, Xue HD. Acta Radiol. 2012;53:1049–1058. doi: 10.1258/ar.2012.120055. [DOI] [PubMed] [Google Scholar]
- 185.Chittasupho C, Lirdprapamongkol K, Kewsuwan P, Sarisuta N. Eur J Pharm Biopharm. 2014;88:529–538. doi: 10.1016/j.ejpb.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 186.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Limon PL, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Science. 2014;346:1450. [Google Scholar]
- 188.He Q, Shi J, Chen F, Zhu M, Zhang L. Biomaterials. 2010;31:3335–3346. doi: 10.1016/j.biomaterials.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 189.Chen Y, Gao Y, Chen HR, Zeng DP, Li YP, Zheng YY, Li FQ, Ji XF, Wang X, Chen F, He QJ, Zhang LL, Shi JL. Adv Funct Mater. 2012;22:1586–1597. [Google Scholar]
- 190.Niu DC, Ma Z, Li YS, Shi JL. J Am Chem Soc. 2010;132:15144–15147. doi: 10.1021/ja1070653. [DOI] [PubMed] [Google Scholar]
- 191.Chen Y, Chu C, Zhou YC, Ru YF, Chen HR, Chen F, He QJ, Zhang YL, Zhang LL, Shi JL. Small. 2011;7:2935–2944. doi: 10.1002/smll.201101055. [DOI] [PubMed] [Google Scholar]
- 192.Coman DR, Delong RP, Mccutcheon M. Cancer Res. 1951;11:648. [PubMed] [Google Scholar]
- 193.Heppner GH. Cancer Res. 1984;44:2259–2265. [PubMed] [Google Scholar]
- 194.Fidler IJ. J Natl Cancer Inst. 1970;45:773. [PubMed] [Google Scholar]
- 195.Miller MC, Doyle GV, Terstappen LW. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, Pierga JY, Farace F, Riethdorf S, Fehm T, Zorzino L, Tibbe AGJ, Maestro M, Gisbert-Criado R, Denton G, de Bono JS, Dive C, Foekens JA, Gratama JW. Cytom Part B-Clin Cy. 2011;80B:112–118. doi: 10.1002/cyto.b.20573. [DOI] [PubMed] [Google Scholar]
- 197.Li FR, Li Q, Zhou HX, Qi H, Deng CY. Nanomed-Nanotechnol. 2013;9:1106–1113. doi: 10.1016/j.nano.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 198.Xu HY, Aguilar ZP, Yang L, Kuang M, Duan HW, Xiong YH, Wei H, Wang A. Biomaterials. 2011;32:9758–9765. doi: 10.1016/j.biomaterials.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim JH, Chung HH, Jeong MS, Song MR, Kang KW, Kim JS. Int J Nanomedicine. 2013;8:2247–2257. doi: 10.2147/IJN.S45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu CH, Huang YY, Chen P, Hoshino K, Liu HY, Frenkel EP, Zhang JXJ, Sokolov KV. ACS Nano. 2013;7:8816–8823. doi: 10.1021/nn403281e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang LL, Zharov VP. Nat Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang X, Qian XM, Beitler JJ, Chen ZG, Khuri FR, Lewis MM, Shin HJC, Nie SM, Shin DM. Cancer Res. 2011;71:1526–1532. doi: 10.1158/0008-5472.CAN-10-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jung SY, Ahn S, Seo E, Lee SJ. J Synchrotron Radiat. 2013;20:324–331. doi: 10.1107/S090904951204873X. [DOI] [PubMed] [Google Scholar]
- 204.Hossain M, Luo Y, Sun ZY, Wang CM, Zhang MH, Fu HY, Qiao Y, Su M. Biosens Bioelectron. 2012;38:348–354. doi: 10.1016/j.bios.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 205.Park GS, Kwon H, Kwak DW, Park SY, Kim M, Lee JH, Han H, Heo S, Li XS, Lee JH, Kim YH, Lee JG, Yang W, Cho HY, Kim SK, Kim K. Nano Lett. 2012;12:2176–2176. doi: 10.1021/nl2045759. [DOI] [PubMed] [Google Scholar]
- 206.Pantel K, Alix-Panabieres C. Trends Mol Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 207.Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JWM, Gratama JW, Sleijfer S, Foekens JA. J Natl Cancer Inst. 2009;101:61–66. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Man Y, WQ, Kemmner W. J Clinic Experiment Pathol. 2011;1:102. [Google Scholar]
- 209.Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Breast Cancer Res. 2008:10. doi: 10.1186/bcr2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ghazani AA, Castro CM, Gorbatov R, Lee H, Weissleder R. Neoplasia. 2012;14:388–395. doi: 10.1596/neo.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, Ke Z, Tseng HR. Acc Chem Res. 2014;47:2941–2950. doi: 10.1021/ar5001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang ST, Liu K, Liu JA, Yu ZTF, Xu XW, Zhao LB, Lee T, Lee EK, Reiss J, Lee YK, Chung LWK, Huang JT, Rettig M, Seligson D, Duraiswamy KN, Shen CKF, Tseng HR. Angew Chem Int Edit. 2011;50:3084–3088. doi: 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang ST, Wang H, Jiao J, Chen KJ, Owens GE, Kamei KI, Sun J, Sherman DJ, Behrenbruch CP, Wu H, Tseng HR. Angew Chem Int Edit. 2009;48:8970–8973. doi: 10.1002/anie.200901668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao LB, Lu YT, Li FQ, Wu K, Hou S, Yu JH, Shen QL, Wu DX, Song M, OuYang WH, Luo Z, Lee T, Fang XH, Shao C, Xu X, Garcia MA, Chung LWK, Rettig M, Tseng HR, Posadas EM. Adv Mater. 2013;25:2897–2902. doi: 10.1002/adma.201205237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hou S, Zhao LB, Shen QL, Yu JH, Ng C, Kong XJ, Wu DX, Song M, Shi XH, Xu XC, OuYang WH, He RX, Zhao XZ, Lee T, Brunicardi FC, Garcia MA, Ribas A, Lo RS, Tseng HR. Angew Chem Int Edit. 2013;52:3379–3383. doi: 10.1002/anie.201208452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hou S, Zhao HC, Zhao LB, Shen QL, Wei KS, Suh DY, Nakao A, Garcia MA, Song M, Lee T, Xiong B, Luo SC, Tseng HR, Yu HH. Adv Mater. 2013;25:1547–1551. doi: 10.1002/adma.201203185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 218.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 219.Rinderknecht M, Detmar M. J Cell Physiol. 2008;216:347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 220.Proulx ST, Detmar M. Exp Cell Res. 2013;319:1611–1617. doi: 10.1016/j.yexcr.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 221.Harrell MI, Iritani BM, Ruddell A. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Galanzha EI. J Blood Lymph. 2011;1:e104. [Google Scholar]
- 223.Akamo Y, Mizuno I, Yotsuyanagi T, Ichino T, Tanimoto N, Yamamoto T, Nagata M, Takeyama H, Shinagawa N, Yura J, Manabe T. Jpn J Cancer Res. 1994;85:652–658. doi: 10.1111/j.1349-7006.1994.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Maincent P, Thouvenot P, Amicabile C, Hoffman M, Kreuter J, Couvreur P, Devissaguet JP. Pharm Res. 1992;9:1534–1539. doi: 10.1023/a:1015895804597. [DOI] [PubMed] [Google Scholar]
- 225.Nishioka Y, Yoshino H. Adv Drug Deliver Rev. 2001;47:55–64. doi: 10.1016/s0169-409x(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 226.Oussoren C, Zuidema J, Crommelin DJA, Storm G. BBA-Biomembranes. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 227.Torchilin VP. Immunomethods. 1994;4:244–258. doi: 10.1006/immu.1994.1027. [DOI] [PubMed] [Google Scholar]
- 228.Kim CK, Jeong EJ. Int J Pharm. 1997;147:143–151. [Google Scholar]
- 229.Kim CK, Choi YJ, Lim SJ, Lee MG, Lee SH, Hwang SJ. Int J Pharm. 1993;98:9–18. [Google Scholar]
- 230.Hawley AE, Illum L, Davis SS. FEBS Lett. 1997;400:319–323. doi: 10.1016/s0014-5793(96)01408-1. [DOI] [PubMed] [Google Scholar]
- 231.Hawley AE, Illum L, Davis SS. Pharm Res. 1997;14:657–661. doi: 10.1023/a:1012117531448. [DOI] [PubMed] [Google Scholar]
- 232.Kaminskas LM, McLeod VM, Ascher DB, Ryan GM, Jones S, Haynes JM, Trevaskis NL, Chan LJ, Sloan EK, Finnin BA, Williamson M, Velkov T, Williams ED, Kelly BD, Owen DJ, Porter CJH. Mol Pharm. 2015;12:432–443. doi: 10.1021/mp500531e. [DOI] [PubMed] [Google Scholar]
- 233.Huang XL, Zhang F, Lee S, Swierczewska M, Kiesewetter DO, Lang LX, Zhang GF, Zhu L, Gao HK, Choi HS, Niu G, Chen XY. Biomaterials. 2012;33:4370–4378. doi: 10.1016/j.biomaterials.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tseng YC, Xu ZH, Guley K, Yuan H, Huang L. Biomaterials. 2014;35:4688–4698. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chambers AF, Groom AC, MacDonald IC. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 236.Joyce JA, Pollard JW. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Paget S. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 238.Loupakis F, Cremolini C, Yang D, Salvatore L, Zhang W, Wakatsuki T, Bohanes P, Schirripa M, Benhaim L, Lonardi S, Antoniotti C, Aprile G, Graziano F, Ruzzo A, Lucchesi S, Ronzoni M, De Vita F, Tonini G, Falcone A, Lenz HJ. PLoS One. 2013;8:e66774. doi: 10.1371/journal.pone.0066774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vonderheide RH, Glennie MJ. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Weiss JM, Ridnour LA, Back T, Hussain SP, He PJ, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH. J Exp Med. 2010;207:2455–2467. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.von Boehmer H, Daniel C. Nat Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 243.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH. Sci Transl Med. 2012;4:134ra162. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Watkins SK, Egilmez NK, Suttles J, Stout RD. J Immunol. 2007;178:1357–1362. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 245.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 246.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu ZY, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Coussens LM, Fingleton B, Matrisian LM. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 248.Kessenbrock K, Plaks V, Werb Z. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xu QM, Guo LC, Gu XH, Zhang BA, Hu X, Zhang JJ, Chen JH, Wang Y, Chen C, Gao B, Kuang YT, Wang SL. Biomaterials. 2012;33:3909–3918. doi: 10.1016/j.biomaterials.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 250.Ni GY, Wang YJ, Wu XL, Wang XF, Chen S, Liu XS. Immunol Lett. 2012;148:126–132. doi: 10.1016/j.imlet.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 251.Zhu YF, Shi JL, Chen HR, Shen WH, Dong XP. Microporous Mesoporous Mater. 2005;84:218–222. [Google Scholar]
- 252.Zhu YF, Shi JL, Li YS, Chen HR, Shen WH, Dong XP. Microporous Mesoporous Mater. 2005;85:75–81. [Google Scholar]
- 253.Zhang J, Yuan ZF, Wang Y, Chen WH, Luo GF, Cheng SX, Zhuo RX, Zhang XZ. J Am Chem Soc. 2013;135:5068–5073. doi: 10.1021/ja312004m. [DOI] [PubMed] [Google Scholar]
- 254.Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Proc Natl Acad Sci U S A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen XL, Li Q, Cao L, Jiang SX. Int J Clin Exp Med. 2014;7:5336–5341. [PMC free article] [PubMed] [Google Scholar]
- 256.Pourtau L, Oliveira H, Thevenot J, Wan YL, Brisson AR, Sandre O, Miraux S, Thiaudiere E, Lecommandoux S. Adv Healthc Mater. 2013;2:1420–1424. doi: 10.1002/adhm.201300061. [DOI] [PubMed] [Google Scholar]
- 257.Peiris PM, Toy R, Doolittle E, Pansky J, Abramowski A, Tam M, Vicente P, Tran E, Hayden E, Camann A, Mayer A, Erokwu BO, Berman Z, Wilson D, Baskaran H, Flask CA, Keri RA, Karathanasis E. ACS Nano. 2012;6:8783–8795. doi: 10.1021/nn303833p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang TZ, Lai VP, Park JO, Ellenbogen RG, Disis ML, Zhang MQ. ACS Nano. 2012;6:2591–2601. doi: 10.1021/nn205070h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Niu DC, Wang X, Li YS, Zheng YY, Li FQ, Chen HR, Gu JL, Zhao WR, Shi JL. Adv Mater. 2013;25:2686–2692. doi: 10.1002/adma.201204316. [DOI] [PubMed] [Google Scholar]
- 260.Rapoport N, Nam KH, Gupta R, Gao ZG, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Parker DL, Jeong EK, Kennedy AM. J Control Release. 2011;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Springer TA. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 262.Springer TA. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 263.Reagan MR, Kaplan DL. Stem Cells. 2011;29:920–927. doi: 10.1002/stem.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Roth JC, Curiel DT, Pereboeva L. Gene Ther. 2008;15:716–729. doi: 10.1038/gt.2008.38. [DOI] [PubMed] [Google Scholar]
- 265.Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, Janes SM. Cancer Res. 2009;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Huang XL, Zhang F, Wang H, Niu G, Choi KY, Swierczewska M, Zhang GF, Gao HK, Wang Z, Zhu L, Choi HS, Lee S, Chen XY. Biomaterials. 2013;34:1772–1780. doi: 10.1016/j.biomaterials.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Huang XL, Zhang F, Wang Y, Sun XL, Choi KY, Liu DB, Choi JS, Shin TH, Cheon J, Niu G, Chen XY. ACS Nano. 2014;8:7549–7549. doi: 10.1021/nn4062726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kim T, Momin E, Choi J, Yuan K, Zaidi H, Kim J, Park M, Lee N, McMahon MT, Quinones-Hinojosa A, Bulte JW, Hyeon T, Gilad AA. J Am Chem Soc. 2011;133:2955–2961. doi: 10.1021/ja1084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhang TY, Huang B, Yuan ZY, Hu YL, Tabata Y, Gao JQ. Nanomed-Nanotechnol. 2014;10:257–267. doi: 10.1016/j.nano.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 270.Lameijer MA, Tang J, Nahrendorf M, Beelen RHJ, Mulder WJM. Expert Rev Mol Diagn. 2013;13:567–580. doi: 10.1586/14737159.2013.819216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ikehara Y, Niwa T, Biao L, Ikehara SK, Ohashi N, Kobayashi T, Shimizu Y, Kojima N, Nakanishi H. Cancer Res. 2006;66:8740–8748. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- 272.Lesimple T, Moisan A, Toujas L. Res Immunol. 1998;149:663–671. doi: 10.1016/s0923-2494(99)80036-4. [DOI] [PubMed] [Google Scholar]
- 273.Jin H, Su JM, Garmy-Susini B, Kleeman J, Varner J. Cancer Res. 2006;66:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 274.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 275.Madsen SJ, Baek SK, Makkouk AR, Krasieva T, Hirschberg H. Ann Biomed Eng. 2012;40:507–515. doi: 10.1007/s10439-011-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Faveeuw C, Trottein F. Cancer Res. 2014;74:1632–1638. doi: 10.1158/0008-5472.CAN-13-3504. [DOI] [PubMed] [Google Scholar]
- 277.Klippstein R, Pozo D. Nanomed-Nanotechnol. 2010;6:523–529. doi: 10.1016/j.nano.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 278.Lum LG. Ca-Cancer J Clin. 1999;49:74–100. doi: 10.3322/canjclin.49.2.74. [DOI] [PubMed] [Google Scholar]
- 279.Paulis LE, Mandal S, Kreutz M, Figdor CG. Curr Opin Immunol. 2013;25:389–395. doi: 10.1016/j.coi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 280.Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 281.Fadel TR, Sharp FA, Vudattu N, Ragheb R, Garyu J, Kim D, Hong EP, Li N, Haller GL, Pfefferle LD, Justesen S, Herold KC, Fahmy TM. Nat Nanotechnol. 2014;9:723–723. doi: 10.1038/nnano.2014.154. [DOI] [PubMed] [Google Scholar]
- 282.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Nat Med. 2010;16:1035–U1135. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bear AS, Kennedy LC, Young JK, Perna SK, Almeida JPM, Lin AY, Eckels PC, Drezek RA, Foster AE. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. Nanoscale Res Lett. 2011:6. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen XY, Wu JC, Sunwoo JB, Cheng Z. Biomaterials. 2012;33:5584–5592. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Guo LM, Zhang LX, Zhang JM, Zhou J, He QJ, Zeng SZ, Cui XZ, Shi JL. Chem Commun. 2009;6071–6073 doi: 10.1039/b911083f. [DOI] [PubMed] [Google Scholar]
- 287.Liu JN, Liu Y, Bu WB, Bu JW, Sun Y, Du JL, Shi JL. J Am Chem Soc. 2014;136:9701–9709. doi: 10.1021/ja5042989. [DOI] [PubMed] [Google Scholar]
- 288.Li LL, Guan YQ, Liu HY, Hao NJ, Liu TL, Meng XW, Fu CH, Li YZ, Qu QL, Zhang YG, Ji SY, Chen L, Chen D, Tang FQ. ACS Nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 289.Cao BR, Yang MY, Zhu Y, Qu XW, Mao CB. Adv Mater. 2014;26:4627–4631. doi: 10.1002/adma.201401550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Duchi S, Sotgiu G, Lucarelli E, Ballestri M, Dozza B, Santi S, Guerrini A, Dambruoso P, Giannini S, Donati D, Ferroni C, Varchi G. J Control Release. 2013;168:225–237. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 291.Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H. J Neurooncol. 2011;104:439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yang TD, Choi W, Yoon TH, Lee KJ, Lee JS, Han SH, Lee MG, Yim HS, Choi KM, Park MW, Jung KY, Baek SK. J Biomed Opt. 2012;17 doi: 10.1117/1.JBO.17.12.128003. [DOI] [PubMed] [Google Scholar]
- 293.Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Int J Cancer. 2005;116:624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 294.Mortensen MW, Kahns L, Hansen T, Sorensen PG, Bjorkdahl O, Jensen MR, Gundersen HJG, Bjornholm T. J Nanosci Nanotechnol. 2007;7:4575–4580. [PubMed] [Google Scholar]
- 295.Cho HR, Choi SH, Lee N, Hyeon T, Kim H, Moon WK. PLoS One. 2012;7:90–99. doi: 10.1371/journal.pone.0029575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang YS, Wang Y, Wang LD, Wang YC, Cai X, Zhang C, Wang LHV, Xia YN. Theranostics. 2013;3:532–543. doi: 10.7150/thno.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhao J, Vykoukal J, Abdelsalam M, Recio-Boiles A, Huang Q, Qiao Y, Singhana B, Wallace M, Avritscher R, Melancon MP. Nanotechnol. 2014;25:405101. doi: 10.1088/0957-4484/25/40/405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Smirnov P. Methods Mol Biol. 2009;512:333–353. doi: 10.1007/978-1-60327-530-9_19. [DOI] [PubMed] [Google Scholar]
- 299.Tavare R, Sagoo P, Varama G, Tanriver Y, Warely A, Diebold SS, Southworth R, Schaeffter T, Lechler RI, Razavi R, Lombardi G, Mullen GED. PLoS One. 2011;6:e19662. doi: 10.1371/journal.pone.0019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sadrieh N. FDA Considerations For Regulation Of Nanomaterial Containing Products. 2006. FDA Docket # 2006-4241B2001-2002-2031. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.