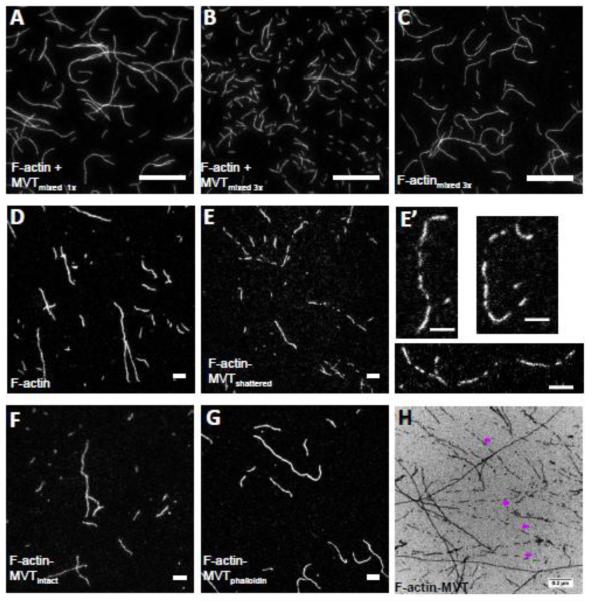
Figure 3. Partial decoration of F-actin with MVT leads to filament fragmentation during sample handling.
A-C) F-actin (1 μM, 20% Cy3b-labeled) was diluted to 25 nM in the presence (A and B) and absence (C) of 0.6 μM MVT (binding density ~0.5) and added to chambers treated with 1% pluronic acid and 0.5 mg/ml casein. Samples with MVT and pipetted three times before addition to the chamber were shorter than those (B) with MVT but pipetted only one time or (C) control samples pipetted three times. Scale bar = 20 μm. D-G) F-actin (2 μM, 20% Cy3b-labeled) was incubated for 2 hours with either 1.6 μM MVT (binding density = 0.5) or MVT storage buffer. Actin samples were diluted to 5 nM in F-buffer supplemented with 100 mM BME containing 0.6 μM MVT in order to maintain a binding density of 0.5 with or without phalloidin. The diluted solutions were spotted onto a glass slide and a poly-L-lysine coated coverslip was placed on top. Scale bars = 5 μm. Representative images are shown for (D) control filaments, (E-F) F-actin-MVT diluted in MVT buffer (different fields from the same sample where filaments appeared fragmented and intact, respectively), and (G) F-actin-MVT diluted in phalloidin buffer. (E’) An enlarged image of fragmented filaments prepared as in (E). See filament length distribution in Supplementary Figure 2. H) Electron micrograph of F-actin (2.5 μM) incubated with equimolar MVT. Pre-polymerized actin was mixed with storage buffer (control not shown) or MVT (H) and spotted on carbon EM grids. As seen in fluorescent images, actin filaments were often fragmented in the presence of MVT as indicated by purple arrows.