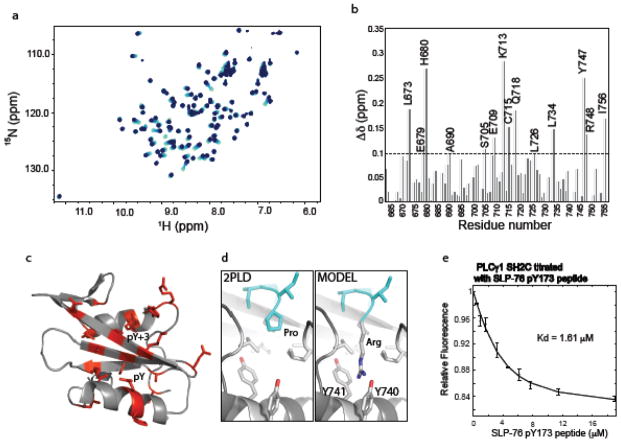
Figure 4.
The SLP-76 pY173 containing peptide binds the PLCγ1 SH2C domain. (a) Superposition of HSQC spectra from the NMR titration of PLCγ1 SH2C with increasing concentration of the SLP-76 pY173 peptide. Spectra are overlaid from light to dark blue corresponding to increasing SLP-76 pY173 peptide concentration. (b) Chemical shift deviations (Δδ) for each of the PLCγ1 SH2C domain residues upon binding of pY173 phosphopeptide. The average deviation (0.1 ppm) is indicated by the horizontal dashed line. (c) Residues for which Δδ > 0.1 ppm are indicated in red on the structure of PLCγ1 SH2C domain. (d) left panel shows the pY+3 pocket in the structure of PLCγ1 SH2C bound to a peptide containing proline at the pY+3 position (PDB ID: 2PLD, peptide ligand is cyan). Right, model of SH2C/peptide complex containing arginine in place of proline in the pY+3 position. Y740 and Y741 are labeled. (e) Fluorescence titration curve of the SLP-76 pY173 phosphopeptide binding to PLCγ1 SH2C.