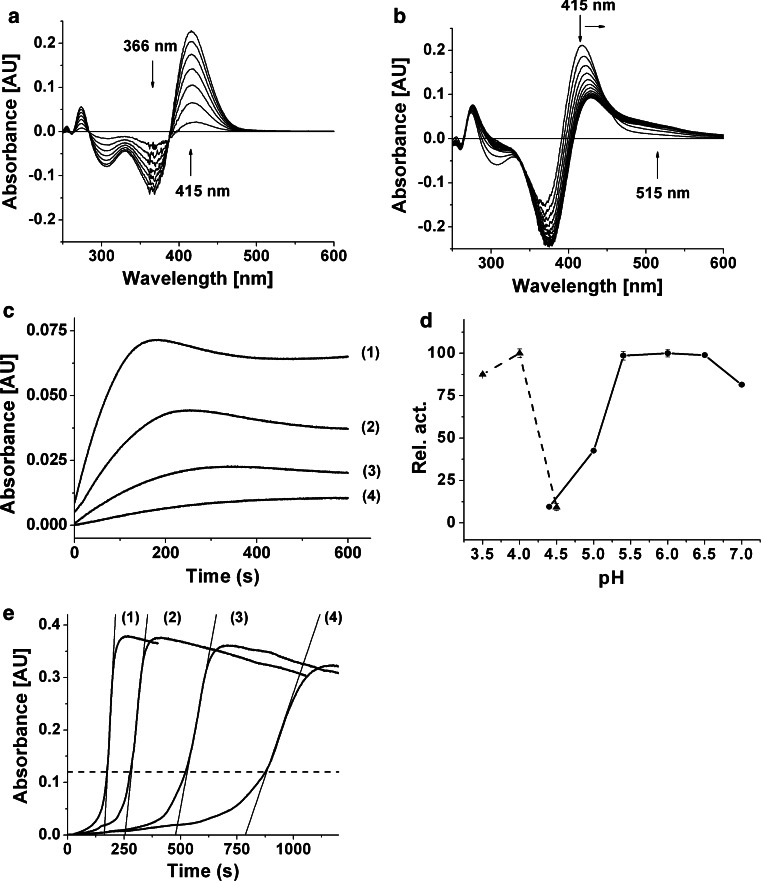
Fig. 6.
Enzymatic properties of latent and active cgAUS1. a, b Difference spectra of the enzymatic oxidation of butein by cgAUS1. The reaction medium contained 25 µm butein in 125 mm sodium citrate buffer, pH 5.5. The final concentration of active cgAUS1 was 0.9 nm. Spectra were recorded every 2 min. a Oxidative conversion of butein to sulfuretin (0–12 min). b Subsequent oxidation of sulfuretin (compare Fig. 8). c Time course of the oxidation of sulfuretin by cgAUS1 monitored at 475 nm indicating suicide inactivation. The reaction medium contained 50 µm sulfuretin in 125 mm sodium citrate buffer, pH 5.5. The final concentrations of active cgAUS1 were 1.2 nm (1), 0.6 nm (2), 0.3 nm (3) and 0.15 nm (4). d pH optimum of recombinant pro-cgAUS1 (filled triangle, dashed lines) and active cgAUS1 (filled circle, solid lines). 25 µm Butein in 125 mm sodium citrate buffers was used over a range from pH 3.5 to 7.4. e Allosteric activation of latent recombinant cgAUS1 monitored at 282 nm. The reaction medium contained 50 µm fisetin in 125 mm sodium citrate buffer, pH 5.5. The final concentrations of latent recombinant cgAUS1 were 7.2 nm (1), 3.6 nm (2), 1.8 nm (3) and 0.9 nm (4). The dashed line depicts the beginning of the steady-state region. The absorbance of 120 mAU corresponds to a product concentration of 23 µm