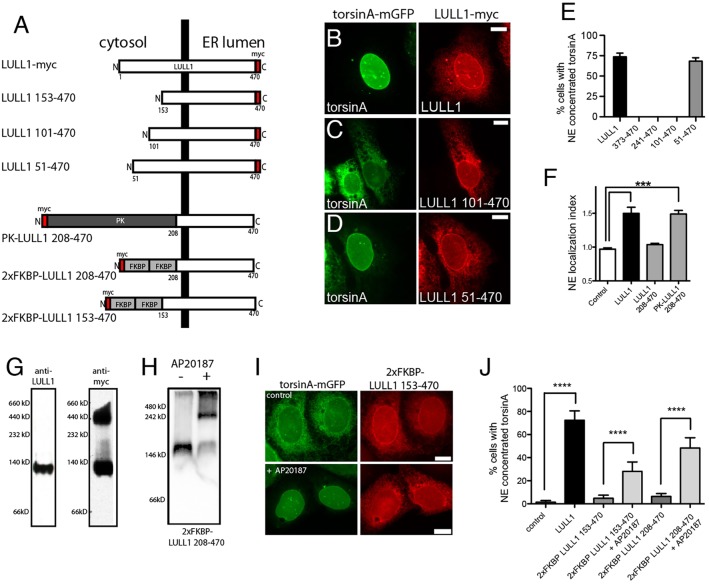
Fig. 6.
The LULL1 cytosolic domain causes LULL1 homo-oligomerization that is required for torsinA relocalization. (A) A series of truncated LULL1 mutants and fusion proteins to study domain function in torsinA relocalization. (B–D) TorsinA is concentrated on the INM of cells co-expressing full-length LULL1 (B) or LULL1 lacking only the first 50 amino acids of the cytosolic domain (D), but remains in the peripheral ER when expressed with LULL1 carrying larger cytosolic domain deletions (C). Images show the localization of GFP fluorescence in U2OS cells stably expressing wild-type torsinA–mGFP (left panels) and transfected with LULL1–Myc proteins (right panels). (E) LULL1-mediated torsinA relocalization requires regions of the LULL1 cytosolic domain. Mean±s.e.m. of the percentage of U2OS cells where wild-type torsinA–mGFP concentrated in the nuclear envelope in response to co-expression of full-length LULL1 or LULL1 deletion mutants. Only the first 50 amino acids of the LULL1 cytosolic domain were unnecessary for torsinA relocalizing activity. (F) A heterologous domain functionally compensates for deletion of the LULL1 cytoplasmic region. Columns show the mean±s.e.m. of the nuclear envelope localization index from n>20 CHO cells transfected with GFP–torsinA-ΔE302/3 and a transmembrane control or LULL1 proteins. ***P<0.001 for the nuclear envelope localization index compared with control (one-way ANOVA with Bonferroni post-hoc analysis). (G) LULL1 overexpression induces high molecular mass LULL1 complexes. Left panel shows that anti-LULL1 western blotting of BN-PAGE-separated U2OS whole-cell lysates detects a single species, and that overexpressed LULL1–Myc (after tetracycline induction) also exists in a higher molecular mass complex. (H) The chemical dimerizer AP20187 induces oligomerization of 2×FKBP LULL1 208-470. Anti-Myc western blotting of CN-PAGE-separated whole-cell lysates from U2OS cells stably expressing torsinA–mGFP and transfected with Myc-tagged 2×FKBP LULL1 208-470, in the absence or presence of the AP20187. (I) Addition of chemical dimerizer induces torsinA relocalization when co-expressed with otherwise inactive 2×FKBP LULL1 153-470. Images show U2OS cells stably expressing torsinA–mGFP (left panels) and transfected with Myc-tagged 2×Dmrb LULL1 153-470 LULL1 (right panels). Although the anti-GFP and Myc signals are both localized in the peripheral ER of untreated cells, AP20187 addition induces torsinA–mGFP relocalization to the INM. (J) The chemical dimerizer AP20187 significantly increases the torsinA-relocalizing activity of FKBP–LULL1 fusion proteins. Columns show the mean±s.e.m of the percentage of U2OS cells with nuclear-envelope-concentrated wild-type torsinA–mGFP in cells expressing either LULL1, 2×FKBP LULL1 153-470 or 2×FKBP LULL1 208-470, and in the presence or absence of AP20187. ****P<0.0001 difference in the percentage of cells with nuclear envelope concentrated torsinA (one-way ANOVA with Bonferroni post-hoc analysis). Scale bars: 10 µm.