Abstract
The aim of this systematic review and meta-analysis was to investigate whether there are any effects of diabetes mellitus on implant failure rates, postoperative infections, and marginal bone loss. An electronic search without time or language restrictions was undertaken in March 2014. The present review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Eligibility criteria included clinical human studies. The search strategy resulted in 14 publications. The I2 statistic was used to express the percentage of total variation across studies due to heterogeneity. The inverse variance method was used for the random effects model when heterogeneity was detected or for the fixed effects model when heterogeneity was not detected. The estimates of an intervention for dichotomous outcomes were expressed in risk ratio and in mean difference in millimeters for continuous outcomes, both with a 95% confidence interval. There was a statistically significant difference (p = .001; mean difference = 0.20, 95% confidence interval = 0.08, 0.31) between diabetic and non-diabetic patients concerning marginal bone loss, favoring non-diabetic patients. A meta-analysis was not possible for postoperative infections. The difference between the patients (diabetic vs. non-diabetic) did not significantly affect implant failure rates (p = .65), with a risk ratio of 1.07 (95% confidence interval = 0.80, 1.44). Studies are lacking that include both patient types, with larger sample sizes, and that report the outcome data separately for each group. The results of the present meta-analysis should be interpreted with caution because of the presence of uncontrolled confounding factors in the included studies.
Keywords: diabetes mellitus, blood glucose, dental implants, infection, periodontal bone loss, meta-analysis
Introduction
Dental implant survival is initially dependent on successful osseointegration following placement. Any alteration of this biological process by excessive surgical trauma, infection, or metabolic upset may adversely affect treatment outcomes (Accursi, 2000). Subsequently, as an implant is restored and placed into function, bone remodeling becomes a critical aspect of implant survival in responding to the functional demands placed on the implant restoration and supporting bone. The critical dependence on bone metabolism for implant survival may be heightened in patients with diabetes (Oates et al., 2013). Diabetes is a chronic disease that occurs when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin that it produces. The number of people with diabetes increased from 153 million (95% uncertainty interval = 127, 182) in 1980 to 347 million (95% uncertainty interval = 314, 382) in 2008 (Danaei et al., 2011). These trends highlight the urgency for a better understanding of diabetes as well as for improving the care of patients with diabetes.
Diabetic patients have increased frequency of periodontitis and tooth loss (Khader et al., 2006), and diabetes has been considered a risky condition for dental implants with the fact that it is associated with delayed wound healing (Rothwell and Richard, 1984), prevalence of microvascular disease (Frantzis et al., 1971), and impaired response to infection (McMahon and Bistrian, 1995). Accordingly, diabetes remains a relative contraindication for implant therapy (Michaeli et al., 2009); that is, well-controlled diabetic patients may be considered appropriate for implant therapy, while diabetic patients lacking good glycemic control may be denied the benefits of implant therapy (Oates et al., 2013). Decreased levels of implant osseointegration have been demonstrated in hyperglycemic animals consistent with untreated type 1 diabetes (Siqueira et al., 2003; de Morais et al., 2009). However, the subject is contradictory, since numerous studies offer indirect evidence for diabetes patients benefiting from oral rehabilitation based on dental implant therapy.
The ability to anticipate outcomes is an essential part of risk management in an implant practice. Recognizing conditions that place the patient at a higher risk of failure will allow the surgeon to make informed decisions and refine the treatment plan to optimize the outcomes (Chrcanovic et al., 2014). The use of implant therapy in special populations requires consideration of potential benefits to be gained from the therapy. To better appreciate this potential, we conducted a systematic review and meta-analysis of published clinical studies to investigate whether dental implant placement in diabetic vs. non-diabetic patients yields any detrimental effects on implant failure rates, postoperative infection, and marginal bone loss. The present study presents a more detailed and profound analysis of the influence of diabetes on implant failure rates previously assessed in a published systematic review (Chrcanovic et al., 2014).
Materials & Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Moher et al., 2009). A review protocol does not exist. For the objective, search strategies, inclusion and exclusion criteria, study selection, and quality assessment, see the Appendix.
Data Extraction and Meta-analysis
From the studies included in the final analysis, the following data were extracted (when available): year of publication, study design, unicenter or multicenter study, number of patients, patients’ age, follow-up, days of antibiotic prophylaxis, mouth rinse, implant healing period, failed and placed implants, postoperative infection, marginal bone loss, and implant surface modification. Contact with authors for possible missing data was performed.
Implant failure and postoperative infection were the dichotomous outcomes measures evaluated. Weighted mean differences were used to construct forest plots of marginal bone loss, a continuous outcome. The statistical unit for “implant failure” and “marginal bone loss” was the implant, and for “postoperative infection,” it was the patient. Whenever outcomes of interest were not clearly stated, the data were not used for analysis. The I2 statistic was used to express the percentage of the total variation across studies due to heterogeneity, with 25%, 50%, and 75% corresponding to low, moderate, and high heterogeneity. The inverse variance method was used for random or fixed effects model. Where statistically significant (p < .10) heterogeneity is detected, a random effects model was used to assess the significance of treatment effects. Where no statistically significant heterogeneity is found, analysis was performed with a fixed effects model (Egger and Smith, 2003). The estimates of an intervention for dichotomous outcomes were expressed in risk ratio and for continuous outcomes in mean difference in millimeters, both with a 95% confidence interval. Only if there were studies with similar comparisons reporting the same outcome measures was meta-analysis attempted.
A funnel plot was drawn (i.e., plot of effect size vs. standard error). Asymmetry of the funnel plot may indicate publication bias and other biases related to sample size, although the asymmetry may also represent a true relationship between trial size and effect size.
The data were analyzed with the statistical software Review Manager (version 5.2.8, Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark, 2014).
Results
For the literature search, see Figure 1 and the Appendix.
Figure 1.
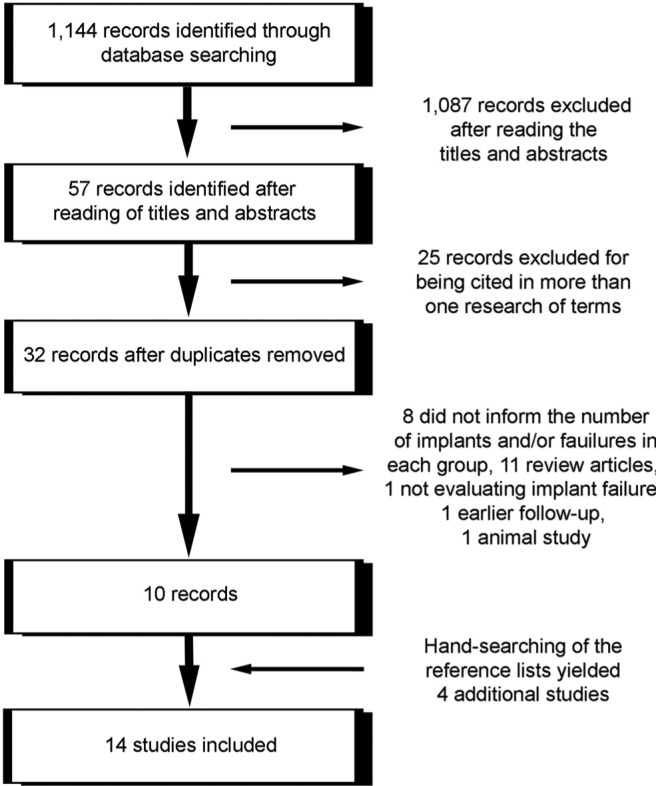
Study screening process.
Description of the Studies
Detailed data of the 14 included studies are listed in Tables 1 and 2. The meta-analysis included 7 controlled clinical trials (Morris et al., 2000; van Steenberghe et al., 2002; Dowell et al., 2007; Alsaadi et al., 2008a; Tawil et al., 2008; Levin et al., 2011; Grandi et al., 2013) and 7 retrospective analyses (Keller et al., 1999; Accursi, 2000; Doyle et al., 2007; Alsaadi et al., 2008b; Anner et al., 2010; Bell et al., 2011; Le et al., 2013). The study of Grandi et al. (2013) was a randomized clinical trial for immediately vs. early loaded implants but not for diabetic vs. non-diabetic patients. Thus, here it was considered a controlled clinical trial.
Table 1.
Detailed Data of the Included Studies
| Implants |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Designa | Patients, n | Age,b yr | Follow-up | Failed / Placed, n | Failure Rate, % | pc | Infectiond |
| Keller et al., 1999 | RA | 54 (2, G1; 52, G2) | 15-73 (28-59) | 12 y | 0 / 11 (G1), 33 / 237 (G2) | 0 (G1), 13.92 (G2) | NM | NM |
| Accursi, 2000 | RA | 45 (15, G1; 30, G2) | 42-83 (57.2, G1), 15-77 (55.7, G2) | 1-17 y | 4 / 59 (G1), 7 / 111 (G2) | 6.78 (G1), 6.31 (G2) | .905 | NM |
| Morris et al., 2000 | CCT | 663 (NM) | NM | 36 mo | 20 / 255 (G1), 180 / 2632 (G2) | 7.84 (G1), 6.84 (G2) | NM | NM |
| van Steenberghe et al., 2002 | CCT | 399 (NM) | 15-80 (50) | NM | 0 / 31 (G1), 27 / 1232 (G2) | 0 (G1), 2.19 (G2) | NM | NM |
| Dowell et al., 2007 | CCT | 35 (25, G1; 10, G2) | 51-81 (NM, G1), 29-61 (45.7, G2) | 4 mo | 0 / 39 (G1), 0 / 11 (G2) | 0 (G1), 0 (G2) | NM | 0 (G1), 0 (G2) |
| Doyle et al., 2007 | RA | 171 (3, G1; 168, G2)e | NM (47.5) | At least 1 yr | 0 / 3 (G1), 12 / 193 (G2)e | 0 (G1), 6.22 (G2) | NM | NM |
| Alsaadi et al., 2008a | CCT | 283 (NM) | 18-86 (56.2) | 6 mo | 2 / 26 (G1), 13 / 694 (G2) | 7.69 (G1), 1.87 (G2) | .02 (type 1), .39 (type 2) | NM |
| Alsaadi et al., 2008b | RA | 412 (10, G1; 402, G2) | NM | ≤2 yr after abutment connection | 0 / 34 (G1), 101 / 1480 (G2) | 0 (G1), 6.82 (G2) | >.05 | NM |
| Tawil et al., 2008 | CCT | 90 (45, G1; 45, G2) | 43-84 (64.7, G1) | M = 42.4 mo (1-12 y) | 6 / 255 (G1), 1 / 244 (G2) | 2.35 (G1), 0.41 (G2) | .66 | 7 (G1), 0 (G2) |
| Anner et al., 2010 | RA | 475 (49, G1; 426, G2) | NM (52) | M = 31 mo (1-114) | 5 / 177 (G1), 72 / 1449 (G2) | 2.82 (G1), 4.97 (G2) | .2076 | NM |
| Bell et al., 2011 | RA | 655 (NM) | NM | M = 20 mo (3-93) | 0 / 83 (G1), 15 / 839 (G2) | 0 (G1), 1.79 (G2) | NM | 8 (G1 + G2) |
| Levin et al., 2011 | CCT | 717 (81, G1; 636, G2) | NM (51) | M = 54 mo (≤114) | 10 / 263 (G1), 83 / 1996 (G2)e | 3.80 (G1), 4.16 (G2) | NM | NM |
| Grandi et al., 2013 | RCTf | 80 (3, G1; 77, G2) | 39-65 (52-55) | 3, 6, 9, 12, 18, 24, 30, 36 mo | 0 / 6 (G1), 0 / 155 (G2)e | 0 (G1), 0 (G2) | NM | NM |
| Le et al., 2013 | RA | 168 (18, G1; 150, G2) | 34-87 (61) | M = 37 mo (21-94) | 2 / 18 (G1), 11 / 203 (G2) | 11.11 (G1), 5.42 (G2) | .32 | NM |
NM, not mentioned; CCT, controlled clinical trial; RCT, randomized controlled trial; RA, retrospective analysis; G1, diabetic patients group; G2, non-diabetic patients group.
All studies are unicenter, except Morris et al. (2000), which is multicenter.
Mean, range.
For failure rate.
Postoperative.
Unpublished information was obtained by personal communication with one of the authors.
The study was an RCT for immediately vs. loaded implants but not for diabetic and non-diabetic patients.
Table 2.
Further Data of Included Studies
| Authors | Antibiotics / Mouth Rinse, d | Healing Period / Loading | Marginal Bone Loss, mm (Mean ± SD) | Diabetes Type (Patients, n) |
|---|---|---|---|---|
| Keller et al. | NM | NM | NM | 2 |
| Modificationa | Turned (Brånemark, Nobel Biocare, Göteborg, Sweden) | |||
| Observations | All implants placed in grafted maxillary sinus or nasal floor with autologous inlay bone; 73 in ex-smokers, 32 in smokers, 11 in patients irradiated | |||
| Accursi | NM | NM | 0.25 ± 0.07 (G1),b 0.06 ± 0.03 (G2)b | 1 (2); 2 (13) |
| Modification | Turned (Brånemark, Nobel Biocare) | |||
| Observations | Smokers: 53.3% (G1), 31.6% (G2) | |||
| Morris et al. | Used, but details were not informed | NM | NM | 2 |
| Modification | Turned (Spectra System, Core-Vent Corporation, DBA Paragon Company, Encino, USA; n = 1,094), HA coated (Spectra System, Core-Vent Corporation; n = 1,793) | |||
| Observations | — | |||
| van Steenberghe et al. | NM | NM | NM | 1 (NM) and 2 (NM) |
| Modification | Turned (Brånemark, Nobel Biocare) | |||
| Observations | Graft in 4 patients, about 12% smokers | |||
| Dowell et al. | 7-10 (G1), 3 (G2) / NM | 4 mo (no load was applied) | NM | 2 |
| Modification | Sandblasted and acid etched (SLA, Straumann, Waldenburg, Switzerland) | |||
| Observations | Implants inserted after at least 4 mo of healing after tooth extraction; no smokers, no grafts | |||
| Doyle et al. | NM | NM | NM | 1 (NM) and 2 (NM) |
| Modification | NM | |||
| Observations | 10 smokers | |||
| Alsaadi et al. | 1 (for 378 implants) / NM | 6 mo (no load was applied) | NM | 1 (NM) and 2 (NM) |
| Modification | Oxidized (Mk III, TiUnite, Nobel Biocare) | |||
| Observations | Implants: 95 in smokers, 9 inserted in fresh extraction sockets | |||
| Alsaadi et al. | NM | NM | NM | 1 (1) and 2 (9) |
| Modification | Turned (Brånemark, Nobel Biocare; n = 1,316), oxidized (Mk III, TiUnite, Nobel Biocare; n = 198) | |||
| Observations | 61 smokers (223 implants) | |||
| Tawil et al. | 7 / 14 | Immediate (58, G1; 59, G2), “conventional” (197, G1; 185, G2) | 0.3 ± 0.5 (G1),c 0.7 ± 0.9 (G1),c 0.21 ± 0.3 (G2) | 2 |
| Modification | Turned (Brånemark, Nobel Biocare; n = 75, G1; n = 104, G2), oxidized (TiUnite, Nobel Biocare; n = 180, G1; n = 140, G2) | |||
| Observations | Some implants were placed in fresh extraction, but the exact number was not informed: 62 sinus lift (34, G1; 28, G2), 35 guided bone regeneration (20, G1; 15, G2), 40 smokers (22, G1; 18, G2) | |||
| Anner et al. | NM | NM | NM | NM |
| Modification | NM | |||
| Observations | 63 smokers | |||
| Bell et al. | 1 / 1 | 3 months | NM | NM |
| Modification | Sandblasted and acid etched (SLA, Straumann) | |||
| Observations | All implants placed in fresh extraction sockets: 123 placed in smokers, 24 in patients taking biphosphonates | |||
| Levin et al. | NM | NM | NM | NM |
| Modification | NM | |||
| Observations | 103 smokers | |||
| Grandi et al. | 7 / 10 | Immediate (n = 81), 2 mo (n = 80) | NM | NM |
| Modification | Double acid-etched (JDEvolution, JDentalCare, Modena, Italy) | |||
| Observations | 22 light smokers (less than 10 cigarettes/d) | |||
| Le et al. | NM | NM | NM | NM |
| Modification | Sandblasted and acid etched (SLA, Straumann; n = 163), titanium blasted (Astra Tech, AstraTech AB, Mölndal, Sweden; n = 41), ? (Zimmer Dental, Warsaw, USA; n = 14), acid etched (3i Implant Innovations, Palm Beach Gardens, USA; n = 2), ? (BioHorizons, Birmingham, USA; n = 1) | |||
| Observations | Only short implants (≤9 mm) restored with single-unit nonsplinted restorations: 13 implants placed in smokers, 114 in grafted sites | |||
NM, not mentioned; G1, diabetic patients group; G2, non-diabetic patients group.
Implant surface modification (brand).
First year of implant loading.
Marginal bone loss observed in 2 subgroups of group G1, in this order: bleeding on probing <15% and >15%.
From the studies with available data of patients’ age, 3 included nonadult patients (Keller et al., 1999; Accursi, 2000; van Steenberghe et al., 2002). Three studies did not inform of the patients’ ages (Morris et al., 2000; Alsaadi et al., 2008b; Bell et al., 2011). Four studies included only patients with diabetes type 2 (Keller et al., 1999; Morris et al., 2000; Dowell et al., 2007; Tawil et al., 2008); 5 studies included patients with type 1 and type 2 diabetes (Accursi, 2000; van Steenberghe et al., 2002; Doyle et al., 2007; Alsaadi et al., 2008a; Alsaadi et al., 2008b); and 4 studies did not offer such information (Anner et al., 2010; Bell et al., 2011; Levin et al., 2011; Le et al., 2013). Two studies (Dowell et al., 2007; Tawil et al., 2008) provided information about the patients’ glycemic control through the estimation of glycosylated hemoglobin (HbA1c). Only 2 studies (Accursi, 2000; Tawil et al., 2008) provided information about marginal bone loss. Three studies provided information about postoperative infection (Dowell et al., 2007; Tawil et al., 2008; Bell et al., 2011), with 15 occurrences among 780 patients receiving 1,471 implants. In one study (Bell et al., 2011), all implants were inserted in fresh extraction sockets, whereas another one (Le et al., 2013) inserted only short implants (≤9 mm) restored with single-unit nonsplinted restorations, and in one study (Keller et al., 1999), all implants were placed in grafted maxillary sinus or nasal floor with autologous inlay bone. Two studies (Dowell et al., 2007; Alsaadi et al., 2008a) had a follow-up to 6 mo; 6 studies had a follow-up of at least 1 yr (Keller et al., 1999; Accursi, 2000; Morris et al., 2000; Doyle et al., 2007; Alsaadi et al., 2008b; Grandi et al., 2013); 5 studies (Tawil et al., 2008; Anner et al., 2010; Bell et al., 2011; Levin et al., 2011; Le et al., 2013) had follow-ups ranging from a mean of 20 to 54 mo, whereas 1 study (van Steenberghe et al., 2002) did not inform of the follow-up period.
Not every article provided information about the number of failed implants by group. Unpublished information concerning the number of failed implants in each group was obtained by personal communication with 1 of the authors in 2 studies (Doyle et al., 2007; Levin et al., 2011). From the 14 studies, a total of 1,260 dental implants were inserted in diabetic patients, with 49 failures (3.89%), and 11,476 implants were inserted in non-diabetic patients, with 555 failures (4.84%). Six studies (Keller et al., 1999; Morris et al., 2000; van Steenberghe et al., 2002; Doyle et al., 2007; Bell et al., 2011; Levin et al., 2011) did not inform whether there was a statistically significant difference or not for the implant failure rates between non-diabetic and diabetic patients, whereas the other 5 studies (Accursi, 2000; Alsaadi et al., 2008b; Tawil et al., 2008; Anner et al., 2010; Le et al., 2013) did not find a statistically significant difference. One study (Alsaadi et al., 2008a) compared the implant failure rates for the non-diabetic patients with the type 1 and type 2 diabetic patients separately, with statistical and nonstatistical significant difference, respectively. There were no implant failures in 2 studies (Dowell et al., 2007; Grandi et al., 2013).
Two studies (Dowell et al., 2007; Tawil et al., 2008) included patients lacking acceptable glycemic control (through the estimation of glycosylated hemoglobin—HbA1c). Dowell et al. (2007) defined poorly controlled type 2 diabetes mellitus in patients having a HbA1c level >10.0%, whereas Tawil et al., (2008) defined it as HbA1c level >9%. Five studies (Keller et al., 1999; van Steenberghe et al., 2002; Alsaadi et al., 2008a; Alsaadi et al., 2008b; Anner et al., 2010) reported patients’ diabetes as “under control” but did not mention the level of control, whereas 1 study (Doyle et al., 2007) informed that assessment of diabetes control was not performed, and 4 studies (Accursi, 2000; Morris et al., 2000; Bell et al., 2011; Le et al., 2013) did not mention any information about glycemic control.
Quality Assessment
Each trial was assessed for risk of bias, and the scores are summarized in Table 3. All studies were judged to be at high risk of bias.
Table 3.
Results of the Quality Assessment
| Study | Incomplete Outcome Data Addressed |
|---|---|
| Keller et al., 1999 | Yes |
| Accursi, 2000 | Yes |
| Morris et al., 2000 | No |
| van Steenberghe et al., 2002 | No |
| Dowell et al., 2007 | Yes |
| Doyle et al., 2007 | No |
| Alsaadi et al., 2008a | Yes |
| Alsaadi et al., 2008b | No |
| Tawil et al., 2008 | No |
| Anner et al., 2010 | No |
| Bell et al., 2011 | No |
| Levin et al., 2011 | Yes |
| Grandi et al., 2013a | No |
| Le et al., 2013 | No |
For all studies, sequence generation was not randomized; allocation concealment was inadequate; there was no blinding; and the estimated potential risk of bias was high.
The study was a randomized clinical trial for immediately vs. loaded implants but not for diabetic and non-diabetic patients.
Meta-analysis
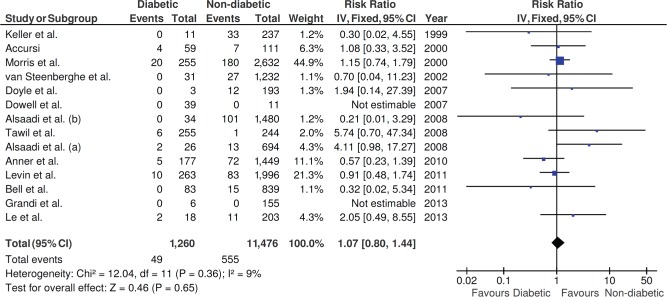
The insertion of dental implants in diabetic or non-diabetic patients did not statistically affect the implant failure rates (p = .65, risk ratio = 1.07, 95% confidence interval = 0.80, 1.44; heterogeneity: I2 = 9%, p = .36, fixed effects model; Figure 2).
Figure 2.
Forest plot for the event “implant failure.”
Three studies (Dowell et al., 2007; Tawil et al., 2008; Bell et al., 2011) provided information about postoperative infection; however, only 2 (Dowell et al., 2007; Tawil et al., 2008) informed of the number of occurrences separated by group. As only 1 (Dowell et al., 2007) of these 2 studies observed occurrences of postoperative infection, a meta-analysis was not possible for this outcome.
Two studies provided information about the marginal bone loss with standard deviation, necessary for the calculation of comparisons in continuous outcomes. There was a statistically significant difference (p = .001, mean difference = 0.20, 95% confidence interval = 0.08, 0.31; random effects model, I2 = 81%, p < .005; Figure 3) between diabetic and non-diabetic patients concerning the marginal bone loss, favoring non-diabetic patients.
Figure 3.
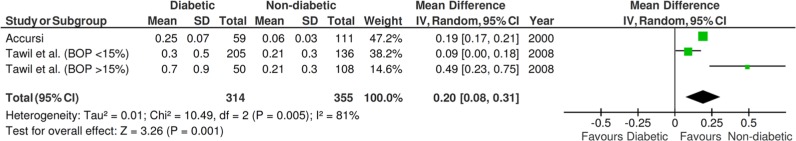
Forest plot for the event “marginal bone loss.” BOP, bleeding on probing.
Publication Bias
The funnel plot did not show asymmetry when the studies reporting “implant failure” were analyzed (Figure 4), indicating absence of publication bias.
Figure 4.
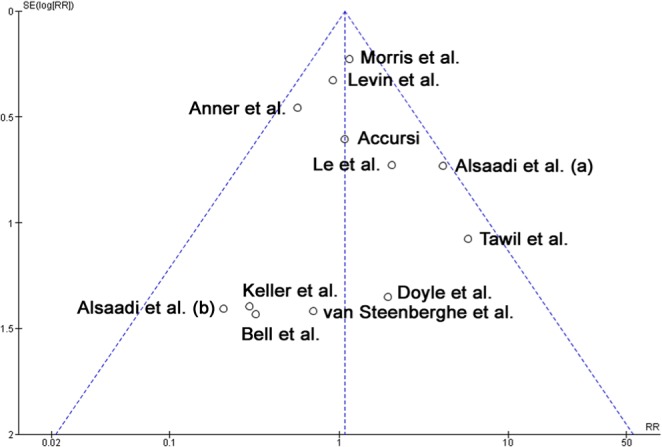
Funnel plot for the studies reporting the outcome event “implant failure.”
Discussion
It has been suggested that the relative contraindication for implant surgery is related to the stability of the diabetic patient’s blood sugar level. Unfortunately, the application of the finding from many studies to clinical practice is limited by the lack of specific information characterizing the patient’s diabetic status. While most of these studies describe the participants’ diabetes as being “well controlled,” the authors do not report how they assessed glycemic control (Dowell et al., 2007). Of the 14 studies included in the present review, only 2 (Dowell et al., 2007; Tawil et al., 2008) provided true valuable information about the patients’ glycemic control, through the estimation of glycosylated hemoglobin (HbA1c). Five studies (Keller et al., 1999; van Steenberghe et al., 2002; Alsaadi et al., 2008a; Alsaadi et al., 2008b; Anner et al., 2010) reported that the patients’ diabetes was under control, but they did not mention the level of control, whereas the other studies did not provide any information about glycemic control. In the study of Moy et al. (2005), even patients with controlled diabetes were almost 3 times as likely to develop implant failure compared with other patients. Unfortunately, the study did not indicate the number of implants in each group, and the failure rates were based on the number of patients in each group; therefore, its data could not be included in the present meta-analysis. Glycemic control is a primary consideration for patients with diabetes, and there is a clear correlation between glycemic control and the development of microvascular and macrovascular complications (Cohen and Horton, 2007). Tissue hyperglycemia affects every aspect of wound healing by adversely affecting the immune system, including neutrophil and lymphocyte function, chemotaxis, and phagocytosis (Goodson and Hunt, 1984). This also leads to a greater predisposition to infection of the wound. Moreover, animal studies showed negative effects of hyperglycemia, not only on bone formation, but also on bone strength and fracture healing (Lu et al., 2003; Siqueira et al., 2003; Kayal et al., 2007). These effects are suggested to affect the osseointegration. However, a prospective evaluation of 58 patients with presumably well-controlled diabetes who received mandibular implants reported that glycemic control was not significantly related to implant success over 5 yr (Olson et al., 2000). Dowell et al. (2007), and Tawil et al. (2008) also observed that compromises in glycemic control may not affect implant success in humans.
Heterogeneity in eligibility criteria for implantation in different diabetic populations may explain the wide between-study variations. The true differences in metabolic effects between type 1 and type 2 diabetes remain unclear (Oates et al., 2013). It has been proposed that diabetes leads to decreased bone turnover, with reductions in both resorption and formation, and that it is the difference in ages of onset of types 1 and 2 diabetes relative to bone growth patterns that leads to these distinctions in outcomes (Krakauer et al., 1995). Because type 1 has an earlier onset than type 2 diabetes, one can assume that implant loss is more frequent in patients with the former form of diabetes. One possible reflection in oral implantology was observed by Alsaadi et al. (2008a), who detected a significant effect of diabetes type 1 on early implant failures (p = .02), with the same not happening with diabetes type 2 (p = .39). However, it is important to observe that in the study of Alsaadi et al. (2008a), only 1 implant was placed in the only patient with diabetes type 1 in the study and this implant failed, whereas 25 implants were inserted in patients with diabetes type 2, with only 1 failure (694 implants were placed in non-diabetic patients, with 13 failures). Prevalence is one of the possible problems in including patients with type 1 diabetes in dental implant studies: >90% of people with diabetes have type 2. Since implant outcomes for patients with type 1 diabetes may differ from those for patients with type 2 diabetes, it is important for studies that include both patient types to report the outcome data separately for each group (Klokkevold and Han, 2007). Thus, it is important to stress that as type 1 and 2 diabetes could have different responses to implant therapy, depending on their level of control, evaluating these 2 conditions together adds an uncontrolled variable to the present meta-analysis.
Animal studies have shown that uncontrolled diabetes hinders bone formation, bone remodeling, and wound healing (Nevins et al., 1998) and causes reduction in bone-to-implant contact (BIC) and bone thickness (Takeshita et al., 1998), while insulin upregulates bone formation (Siqueira et al., 2003) and maintains BIC (Kwon et al., 2005). The effects of a hyperglycemic state have been shown to include inhibition of osteoblastic cell proliferation and collagen production during the early stages of callus development, resulting in reduced bone formation as well as diminished mechanical properties of the newly formed bone (Lu et al., 2003). Reduced BIC may indicate a poorer healing response and may predict a reduced ability of the implant to withstand bacterial and load challenges. If the lack of BIC is carried to the extreme, osseointegration would be deemed to have failed, and the implant would be found to be mobile at stage 2 surgery (Accursi, 2000). Oates et al. (2009) demonstrated alterations in implant stability consistent with impaired implant integration for persons with type 2 diabetes mellitus in direct relation to hyperglycemic conditions. It was observed that persons with HbA1c ≥8.1% had a greater maximum decrease in stability from baseline and required a longer time for healing, which also suggests alterations in the biological integration of the implants in direct relation to glycemic control (Oates et al., 2009). It seems reasonable to postulate that an implant demonstrating reduced BIC may be less able to withstand functional stresses placed on it during the healing phase. Further breakdown of the peri-implant bone could therefore ensue, and this could result in a loosening of the implant and its ultimate failure (Accursi, 2000). However, this has not yet been clinically proved, since most studies here reviewed did not indicate the times for which the implants placed in diabetic patients were loaded. The study of Tawil et al. (2008) was the only one in which some implants (n = 58) inserted in diabetic patients were submitted to immediate loading, but no failure was observed after a mean follow-up of 42 mo.
With respect to marginal bone loss, we did find a significant difference in favor of non-diabetic patients, with less marginal bone loss than diabetic ones. However, one has to observe that the difference was based on only 2 publications and that marginal bone loss is part of some criteria for success (Albrektsson et al., 1986) but not of others (Buser et al., 1990). In the light of a current and very active discussion of reasons for marginal bone loss and subsequent potential development of peri-implantitis, we find it relevant to report on the difference found, even if precise clinical conclusions may be difficult to draw at present.
Because of the small sample size in some studies (Accursi, 2000; Dowell et al., 2007; Tawil et al., 2008), no definite conclusions on implant survival can be drawn. Moreover, many studies (Doyle et al., 2007; Alsaadi et al., 2008a; Alsaadi et al., 2008b; Anner et al., 2010; Levin et al., 2011; Le et al., 2013) had a much smaller number of diabetic patients in comparison with the number of non-diabetic patients. Even though the importance of meta-analyses is to increase the sample size of individual trials to reach more precise estimates of the effects of interventions, in this particular analysis no statistically significant difference was found when implant failure rates were compared in diabetic and non-diabetic patients (p = .65). These discordant results may demonstrate our continuing need to clarify the parameters of diabetes affecting successful implant therapy.
In 2 studies (Dowell et al., 2007; Alsaadi et al., 2008a), the patients were followed for a short period (up to 6 mo). Thus, even though it is especially during the healing time, up to abutment surgery, that systemic factors can be most easily identified—as other risk factors that occur after abutment surgery do not apply (van Steenberghe et al., 2003)—only early failures could be assessed. A longer follow-up period can lead to an increase in the failure rate. Moreover, the results found in the studies differed from one another, and this difference could be due to factors such as differences in the patients included in the study or the clinicians placing and restoring the implants. For example, Olson et al. (2000) observed that implant failure had a statistically significant association with an increase in years of diabetic history. The authors hypothesized that as duration of diabetes is associated with increased classic microvascular complications, this increase in microvascular disease may be postulated to have contributed to implant failure. However, Tawil et al. (2008) divided the patients with well-controlled diabetes into 4 groups (with reference to duration of diabetes), and the results showed no significant differences in implant survival rates among them.
The study of Morris et al. (2000) was the only one associating some variables to diabetes and implant failure rates. They reported improved implant survival for patients who were treated with antibiotics in comparison with those treated without prophylactic antibiotics, but the survival improvement was greater in diabetic patients (97.1% vs. 86.6%) than in non-diabetic patients (95.1% vs. 90.6%). The same happened in diabetic and non-diabetic patients when the use or nonuse of chlorhexidine rinses was evaluated.
The use of grafting in some studies (Keller et al., 1999; van Steenberghe et al., 2002; Tawil et al., 2008; Le et al., 2013) is a confounding risk factor, as well as the presence of some smokers among the patients (Keller et al., 1999; Accursi, 2000; van Steenberghe et al., 2002; Doyle et al., 2007; Alsaadi et al., 2008a; Alsaadi et al., 2008b; Tawil et al., 2008; Anner et al., 2010; Bell et al., 2011; Levin et al., 2011; Grandi et al., 2013; Le et al., 2013), some patients taking biphosphonates (Bell et al., 2011), insertion of some implants (Alsaadi et al., 2008a; Tawil et al., 2008) or all (Bell et al., 2011) in fresh extraction sockets, insertion of only short implants (Le et al., 2013), and the insertion of implants from different brands and surface treatments. Titanium with different surface modifications shows a wide range of chemical/physical properties and surface topographies/morphologies, depending on how they are prepared and handled (Chrcanovic et al., 2012; Chrcanovic et al., 2013), and it is not clear whether, in general, one surface modification is better than another (Wennerberg and Albrektsson, 2009; Wennerberg and Albrektsson, 2010). These variables may have affected the outcome—and not just the subjection of the insertion of implants in patients who had diabetes or not. The impact of these variables on implant survival rate is difficult to estimate if these factors are not identified separately between the 2 different procedures (i.e., to perform meta-regression analysis). A greater level of statistical significance might have been realized had the confounding variables not been present.
These findings must be viewed as preliminary in that they include relatively small numbers of patients having elevated glycemic levels and they offer only limited information on the longer term effects of diabetes on implant survival. It is also important to consider the potential for many other factors, such as technological advances in implant designs to enhance survival rates for implants in patients with diabetes.
The results of the present study should be interpreted with caution because of its limitations. First of all, all uncontrolled confounding factors may have affected the long-term outcomes and not just the fact that the implants were inserted in either diabetic or non-diabetic patients; the impact of these variables on implant survival rate, postoperative infection, and marginal bone loss is difficult to estimate if these factors are not identified separately in order to perform a metaregression analysis. The lack of control of the confounding factors limited the potential to draw robust conclusions. Unfortunately, most available data regarding diabetes as a risk factor in implant dentistry are extracted from case series. Because of conflicting data from studies with small sample sizes or case series, from groups that were not completely comparable at baseline in some studies, or from studies involving multiple surgeons, clinicians are unable to provide concrete answers to questions posed by patients seeking dental implant treatment. Second, some of the included studies had a retrospective design, and the nature of a retrospective study inherently results in flaws. These problems were manifested by the gaps in information and incomplete records. Furthermore, all data rely on the accuracy of the original examination and documentation. Items may have been excluded in the initial examination or not recorded in the medical chart (Chrcanovic et al., 2010a; Chrcanovic et al., 2010b).
For a more definite conclusion, we believe that future controlled studies with a larger number of patients in the diabetic group are required to determine the real effect of the condition on the dental implant outcome (i.e., most studies included far fewer diabetic than non-diabetic patients).
Conclusion
The results of the present systematic review should be interpreted with caution because of the presence of uncontrolled confounding factors in the included studies. Within the limits of the existing investigations, the difference between the insertion of dental implants in non-diabetic and diabetic patients did not statistically affect the implant failure rates. However, the studies in the review show heterogeneity in eligibility criteria for implantation in different diabetic populations. Studies are lacking that include both patient types, with larger sample sizes, and that report outcome data separately for each group.
Supplementary Material
Acknowledgments
The authors thank Dr. Tommaso Grandi for having sent us his article and Dr. James S. Hodges, Dr. Ronen Ofec, Dr. Liran Levin, and Dr. Tommaso Grandi, who provided us some missing information about their studies.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Accursi GE. (2000). Treatment outcomes with osseointegrated Brånemark implants in diabetic patients: a retrospective study (thesis). Toronto, ON: University of Toronto. [Google Scholar]
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. (1986). The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants 1:11-25. [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Michiles K, Teughels W, Komárek A, van Steenberghe D. (2008a). Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J Clin Periodontol 35:51-57. [DOI] [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. (2008b). Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19:670-676. [DOI] [PubMed] [Google Scholar]
- Anner R, Grossmann Y, Anner Y, Levin L. (2010). Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival: a long-term retrospective evaluation of patients followed for up to 10 years. Implant Dent 19:57-64. [DOI] [PubMed] [Google Scholar]
- Bell CL, Diehl D, Bell BM, Bell RE. (2011). The immediate placement of dental implants into extraction sites with periapical lesions: a retrospective chart review. J Oral Maxillofac Surg 69:1623-1627. [DOI] [PubMed] [Google Scholar]
- Buser D, Weber HP, Lang NP. (1990). Tissue integration of nonsubmerged implants: 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin Oral Implants Res 1:33-40. [DOI] [PubMed] [Google Scholar]
- Chrcanovic BR, Abreu MH, Freire-Maia B, Souza LN. (2010a). Facial fractures in children and adolescents: a retrospective study of 3 years in a hospital in Belo Horizonte, Brazil. Dent Traumatol 26:262-270. [DOI] [PubMed] [Google Scholar]
- Chrcanovic BR, Souza LN, Freire-Maia B, Abreu MH. (2010b). Facial fractures in the elderly: a retrospective study in a hospital in Belo Horizonte, Brazil. J Trauma 69:E73-E78. [DOI] [PubMed] [Google Scholar]
- Chrcanovic BR, Pedrosa AR, Martins MD. (2012). Chemical and topographic analysis of treated surfaces of five different commercial dental titanium implants. Mater Res 15:372-382. [Google Scholar]
- Chrcanovic BR, Leão NLC, Martins MD. (2013). Influence of different acid etchings on the superficial characteristics of Ti sandblasted with Al2O3. Mater Res 16:1006-1014. [Google Scholar]
- Chrcanovic BR, Albrektsson T, Wennerberg A. (2014). Reasons for failures of oral implants. J Oral Rehabil 41:443-476. [DOI] [PubMed] [Google Scholar]
- Cohen A, Horton ES. (2007). Progress in the treatment of type 2 diabetes: new pharmacologic approaches to improve glycemic control. Curr Med Res Opin 23:905-917. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. (2011). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination survey and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31-40. [DOI] [PubMed] [Google Scholar]
- de Morais JA, Trindade-Suedam IK, Pepato MT, Marcantonio E, Jr., Wenzel A, Scaf G. (2009). Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: a digital subtraction radiography study in rats. Clin Oral Implants Res 20:796-801. [DOI] [PubMed] [Google Scholar]
- Dowell S, Oates TW, Robinson M. (2007). Implant success in people with type 2 diabetes mellitus with varying glycemic control: a pilot study. J Am Dent Assoc 138:355-361. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Hodges JS, Pesun IJ, Baisden MK, Bowles WR. (2007). Factors affecting outcomes for single-tooth implants and endodontic restorations. J Endod 33:399-402. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD. (2003). Principles of and procedures for systematic reviews. In: Systematic reviews in health care: meta-analysis in context. Egger M, Smith GD, Altman DG, editors. London, UK: BMJ Books, pp. 23-42. [Google Scholar]
- Frantzis TG, Reeve CM, Brown AL., Jr (1971). The ultrastructure of capillary basement membranes in the attached gingiva of diabetic and non-diabetic patients with periodontal disease. J Periodontol 42:406-411. [DOI] [PubMed] [Google Scholar]
- Goodson WH, Hunt TK. (1984). Wound healing in well-controlled diabetic men. Surg Forum 35:614-616. [Google Scholar]
- Grandi T, Guazzi P, Samarani R, Grandi G. (2013). A 3-year report from a multicentre randomised controlled trial: immediately versus early loaded implants in partially edentulous patients. Eur J Oral Implantol 6:217-224. [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, et al. (2007). Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EE, Tolman DE, Eckert SE. (1999). Maxillary antral-nasal inlay autogenous bone graft reconstruction of compromised maxilla: a 12-year retrospective study. Int J Oral Maxillofac Implants 14:707-721. [PubMed] [Google Scholar]
- Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. (2006). Periodontal status of diabetics compared with non-diabetics: a meta-analysis. J Diabetes Complications 20:59-68. [DOI] [PubMed] [Google Scholar]
- Klokkevold PR, Han TJ. (2007). How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Implants 22(Suppl):173-202. [PubMed] [Google Scholar]
- Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. (1995). Bone loss and bone turnover in diabetes. Diabetes 44:775-782. [DOI] [PubMed] [Google Scholar]
- Kwon PT, Rahman SS, Kim DM, Kopman JA, Karimbux NY, Fiorellini JP. (2005). Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J Periodontol 76:621-626. [DOI] [PubMed] [Google Scholar]
- Le BT, Follmar T, Borzabadi-Farahani A. (2013). Assessment of short dental implants restored with single-unit nonsplinted restorations. Implant Dent 22:499-502. [DOI] [PubMed] [Google Scholar]
- Levin L, Ofec R, Grossmann Y, Anner R. (2011). Periodontal disease as a risk for dental implant failure over time: a long-term historical cohort study. J Clin Periodontol 38:732-737. [DOI] [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT. (2003). Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144:346-352. [DOI] [PubMed] [Google Scholar]
- McMahon MM, Bistrian BR. (1995). Host defenses and susceptibility in patients with diabetes mellitus. Infect Dis Clin North Am 9:1-9. [PubMed] [Google Scholar]
- Michaeli E, Weinberg I, Nahlieli O. (2009). Dental implants in the diabetic patient: systemic and rehabilitative considerations. Quintessence Int 40:639-645. [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264-269, W64. [DOI] [PubMed] [Google Scholar]
- Morris HF, Ochi S, Winkler S. (2000). Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol 5:157-165. [DOI] [PubMed] [Google Scholar]
- Moy PK, Medina D, Shetty V, Aghaloo TL. (2005). Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants 20:569-577. [PubMed] [Google Scholar]
- Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. (1998). Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants 13:620-629. [PubMed] [Google Scholar]
- Oates TW, Dowell S, Robinson M, McMahan CA. (2009). Glycemic control and implant stabilization in type 2 diabetes mellitus. J Dent Res 88:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. (2013). A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Impl Res 24:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. (2000). Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants 15:811-818. [PubMed] [Google Scholar]
- Rothwell BR, Richard EL. (1984). Diabetes mellitus: medical and dental considerations. Spec Care Dent 4:58-65. [DOI] [PubMed] [Google Scholar]
- Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. (2003). Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent 12:242-251. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Murai K, Iyama S, Ayukawa Y, Suetsugu T. (1998). Uncontrolled diabetes hinders bone formation around titanium implants in rat tibiae: a light and fluorescence microscopy, and image processing study. J Periodontol 69:314-320. [DOI] [PubMed] [Google Scholar]
- Tawil G, Younan R, Azar P, Sleilati G. (2008). Conventional and advanced implant treatment in the type II diabetic patient: surgical protocol and long-term clinical results. Int J Oral Maxillofac Implants 23:744-752. [PubMed] [Google Scholar]
- van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. (2002). The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res 13:617-622. [DOI] [PubMed] [Google Scholar]
- van Steenberghe D, Quirynen M, Molly L, Jacobs R. (2003). Impact of systemic diseases and medication on osseointegration. Periodontol 2000 33: 163-171. [DOI] [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T. (2009). Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 20(Suppl 4):172-184. [DOI] [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T. (2010). On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants 25:63-74. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.