Abstract
Deep brain stimulation has become a well-established symptomatic treatment for Parkinson's disease during the last 25 years. Besides improving motor symptoms and long-term motor complications, positive effects on patients’ mobility, activities of daily living, emotional well-being and health-related quality of life have been recognized. Apart from that, numerous clinical trials analyzed effects on non-motor symptoms and side effects of deep brain stimulation. Several technical issues and stimulation paradigms have been and are still being developed to optimize the therapeutic effects, minimize the side effects and facilitate handling. This review summarizes current therapeutic issues, i.e., patient and target selection, surgical procedure and programming paradigms. In addition it focuses on neuropsychological effects and side effects of deep brain stimulation.
Keywords: Parkinson's disease, deep brain stimulation, subthalamic nucleus
Introduction
First performed in late 1980s, deep brain stimulation (DBS) of the subthalamic nucleus (STN) and the internal globuspallidus (GPi) or ventral intermedius nucleus (VIM) of the thalamus has developed and become a distinguished symptomatic treatment for Parkinson's disease (PD). Especially DBS of the STN and GPi is an effective option to improve motor symptoms and manage long-term motor complications resulting from levodopa treatment, such as wearing-off phenomena and dyskinesias. Furthermore patients’ mobility, activities of daily living, emotional well-being and health-related quality of life which are impaired by motor symptoms and complications (Damiano et al., 2000; Chapuis et al., 2005; Chaudhuri et al., 2013), can be enhanced by DBS (Volkmann et al., 2001; Deuschl et al., 2006). To further optimize its efficacy technical issues and stimulation paradigms are still being developed. One aim of this review is to summarize current technical issues and stimulation paradigms. The other aim is to give an overview of the clinical effects and side effects of DBS with a focus on neuropsychological aspects.
Clinical Outcome
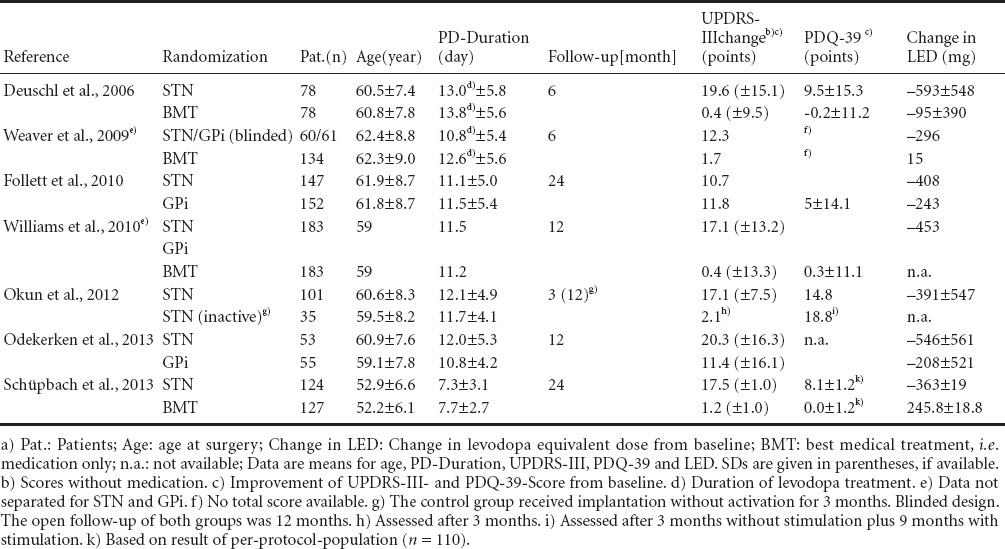
Numerous clinical trials – few of them already providing long-term data between 8 and 10 years of follow-up (Fasano et al., 2010; Castrioto et al., 2011) – could demonstrate an improvement of levodopa-responsive motor symptoms and motor complications, a reduction of the levodopa equivalent dose and an increase in quality of life after DBS (Table 1). These trials provide a high level of evidence namely: Level I–II due to the prospective randomized nature of the trials (Oxford Centre for Evidence-based Medicine – Levels of Evidence; March 2009). However, placebo controlled trials assessing prospectively quality of life are not available for DBS in PD.
Table 1.
Prospective randomized controlled clinical trials of deep brain stimulation (DBS) in Parkinson's disease (PD)a)
Patient Selection
An essential aspect influencing the outcome after DBS in PD is patient selection and timing of surgery. Main indications for DBS in PD patients are levodopa-induced motor fluctuations, dyskinesias and unmanageable tremor. Preoperative indicators for a good outcome are younger age and shorter disease duration, high levodopa-response, few axial motor symptoms, absence of dementia, stable psychiatric conditions and no or non-severe comorbidities (Bronstein et al., 2011). With the exception of non-levodopa-responsive tremor, the preoperative levodopa-response is one of the most important outcome predictors (Charles et al., 2002). Several studies have shown a positive correlation between preoperative levodopa-response and motor improvement (Kleiner-Fisman et al., 2003; Kim et al., 2013).
Regarding cognitive impairment and psychiatric disorders, there are no stringent criteria available (Lang et al., 2006). Dementia is one of the most common exclusion criteria (Bronstein et al., 2011). The management of mild cognitive impairment is still handled more heterogenously. Besides age and levodopa-equivalence dosage the axial subscore, i.e., speech, neck rigidity, posture, rising from chair, gait and postural instability, in the Unified PD Rating Scale (UPDRS) is reported to be a predicting factor for executive dysfunction after DBS (Daniels et al., 2010). Prognostic factors for the development of depressive symptoms after surgery are preoperative persistently increased scores for depression and anxiety measured by the State-Trait Anxiety Inventory (STAI), Beck Depression Inventory (BDI) and Clinical Global Impression - Improvement scale (CGI-I) (Schneider et al., 2010). Therefore depression and other unstable psychiatric conditions require a careful preoperative assessment, a stabilizing treatment and a close-meshed postoperative follow-up (Bronstein et al., 2011).
Concerning time point selection, age, Hoehn and Yahr stage and disease duration are important criteria. There is no clear age cut off, but patients over 70 years have a higher incidence of relevant comorbidities and cognitive impairment resulting in an increased risk for peri- and postoperative complications (Russmann et al., 2004; Lang et al., 2006; Ory-Magne et al., 2007). In the past DBS used to be performed after 11 to 13 years of disease duration in patients with advanced motor-complications (Follett et al., 2010; Williams et al., 2010; Okun et al., 2012). Recent data show high efficacy of DBS concerning an improvement of motor symptoms and quality of life in patients with shorter disease duration and early motor complications (Schüpbach et al., 2013). In this study patients with a mean age of 52 years, a mean PD duration of 7.5 years and motor fluctuations of any severity persisting for 1.7 years were treated with STN-DBS and medication or received best medical treatment. The quality of life - measured by the PD Questionnaire (PDQ-39) - improved by 26% in the stimulation group, whereas it worsened in the medication group. Psychosocial aspects were also analyzed and improved significantly in the stimulation group. The UPDRS motor score improved by 53% in the stimulation group in comparison to 4% in the medication group. These results favor the application of STN-DBS in an earlier stage of PD.
Target Point
Although mechanisms of action of DBS are not completely known up to date, modulation of pathological local (beta) oscillations (Kühn et al., 2008) and modulation of the basal ganglia-cortical network including the hyperdirect pathway seems to play an important role (Gradinaru et al., 2009). The optimal target point for DBS in PD is still a matter of debate. STN-DBS was first performed in 1993 in an advanced PD patient leading to a reduction of motor fluctuations, dyskinesias and dopaminergic medication (Benabid et al., 1994). Since then, several studies have reported an improvement of levodopa responsive symptoms (Limousin et al., 1998; Kleiner-Fisman et al., 2006), motor fluctuations and dyskinesia after STN-DBS (Follett et al., 2010; Okun et al., 2012). STN-DBS is usually performed bilaterally, but unilateral STN-DBS can be effective in highly asymmetric tremor-dominant PD patients (Kumar et al., 1999). Currently, STN-DBS is the most frequently used surgical therapy in PD. However, concerning the improvement of major PD symptoms no significant difference in efficacy has been shown between the STN and the GPi (Weaver et al., 2012), concerning the reduction of dopaminergic medication doses the STN is favorable (Follett et al., 2010; Moro et al., 2010). Furthermore, STN-stimulation requires lower electrical power and results in longer battery life-spans (Volkmann et al., 2001). One advantage of GPi-stimulation is a direct and significant reduction of dyskinesias. Another advantage – in comparison to STN – seems to be a better outcome regarding depression scores (Follett et al., 2010).
In contrast to STN- and GPi-DBS, stimulation of the VIM has no effects on dyskinesia, motor fluctuations, rigidity and bradykinesia but a clear and immediate effect on tremor (Benabid et al., 1996; Ondo et al., 1998). Certainly, VIM-DBS is a therapeutic option for patients with essential tremor and elderly patients with a unilateral tremor-dominant PD.
Another target point is the pedunculopontine nucleus (PPN). DBS of the PPN has been analyzed in small experimental trials. There is evidence revealing that stimulation of the PPN might have positive effects on parkinsonian gait disorder, postural instability and freezing (Pereira et al., 2008). Other reported effects concern a modification of vigilance and quality of sleep (Alessandro et al., 2010). Stimulation of the substantia nigra pars reticulata, partially in combination with the STN, remains experimental. An assumed effect on axial motor symptoms could not yet be proved, but an improvement of freezing of gait has been observed (Weiss et al., 2013).
Effects on Cognition, Behavior and Mood
Changes of neurocognitive function, behavior and mood after DBS in PD have been described in several studies with partially conflicting results. Recent data suggest that the most frequent cognitive side effect, verbal fluency deficits, may be caused by surgical implantation (Okun et al., 2012). In this study, 136 patients underwent DBS device implantation, whereof 101 patients received immediate STN-stimulation and 35 received stimulation after 3 months. Verbal fluency, measured by Delis-Kaplan Executive Function System, degraded similarly in both groups without further aggravation after 3 months. Witt et al. (2013) observed a decline in Mattis Dementia Rating Scale in 7 out of 31 patients with STN-DBS. In comparison to patients without cognitive impairment lead trajectories in these 7 patients harmed a significantly larger volume of the caudate nucleus. However, there is also evidence that stimulation itself has an effect on verbal fluency: Low-frequency (10 Hz) STN-DBS has been shown to improve verbal fluency in comparison to higher stimulation frequencies (130 Hz) and no stimulation (Wojtecki et al., 2006).
Also other factors of stimulation intensity, the localization of the electrode and respective volume of tissue activated impact verbal fluency (Mikos et al., 2011). Regarding speech performance the cognitive (executive-function) aspect has to be clearly distinguished from the voice/articulation/loudness aspect that can be ameliorated or deteriorated by DBS depending on factors such as preoperative speech impairment (Tripoliti et al., 2008; Astrom et al., 2010; Skodda et al., 2012, 2014).
Regarding depression, there are some clinical trials reporting an improvement after STN-DBS (Witt et al., 2008; Okun et al., 2012). One explanation for these results might be an increase in quality of life and reduction of motor symptoms and complications. Others revealed a beneficial effect of GPi-DBS on depression and a worsening effect of STN-DBS (Odekerken et al., 2013). The deteriorating effect of STN-DBS might be caused by a reduction of dopaminergic medication. Apart from that, disease progression has to be taken into account as well (Houeto et al., 2002; Follett et al., 2010). Furthermore, there are reports of a detrimental effect of STN-DBS on fatigue. Okun et al. (2012) described that STN-stimulation rather than the surgical procedure appears to be responsible for this side-effect. In comparison to PD patients with STN-stimulation, their control group received implantation without stimulation for three months. Only stimulation of the STN was associated with fatigue.
Impulse control disorders – typically caused by dopamine agonists – are expected to improve after STN-DBS, mainly due to reduction of dopaminergic medication (Demetriades et al., 2011). Nevertheless, there are studies reporting regression, new development or persistence of impulse control disorders (Smeding et al., 2007; Knobel et al., 2008; Lim et al., 2009).
Surgical Aspects
Target point localization is attained by preoperative imaging and intraoperative neurophysiology. Most frequently, stereotactic magnetic resonance imaging (MRI) is used for target identification and target coordinates are calculated relative to the stereotactic frame placed on the patient's head (Dormont et al., 2010). Further options apart from direct targeting are fusion of MRI and computed tomography (Liu et al., 2001) and stereotactic ventriculography, which is still but rarely used by some teams (Benabid et al., 2009).
Intraoperative neurophysiology consists of intraoperative microelectrode recording (MER) and test stimulation and is used to improve targeting accuracy. For MER multiple trajectories can be recorded simultaneously or successively (Benabid et al., 2009). MER of the STN is characterized by typical activity patterns, proprioceptive responses to passive movements and asymmetrical spikes at high frequency in a bursting manner (Benabid et al., 2009). Some studies suggest a significantly better clinical outcome after microelectrode recording (Mann et al., 2009; Reck et al., 2012), but longer surgery duration has to be taken into account. Intraoperative test stimulation can be performed under both local anesthesia and general anesthesia. However, local anesthesia allows a communication with the patient during the operation and thereby a more precise assessment of side effects and the effect on a variety of symptoms, i.e., rigidity, tremor, coordination and speech. In contrast, general anesthesia is a helpful option to reduce patient's stress and pain (Lefaucheur et al., 2008; Benabid et al., 2009). After identifying the best track the final lead is implanted and subcutaneously connected to the implantable pulse generator (IPG). Typical sites for the IPG are infraclavicular area and lower abdomen. At present, there are rechargeable and non-rechargeable IPGs available. Depending on stimulation parameters – higher stimulation amplitude and pulse width result in shorter battery life spans (Ondo et al., 2007) – non-rechargeable IPGs need to be replaced after approximately 5 years by surgery and are favored for elderly patients and patients with few technical skills.
Programming
The main aim of programming is reducing motor-symptoms and complications and simultaneously avoiding or minimizing side effects of stimulation.
Stimulation devices of the first generation delivered electrical stimulation in a voltage-controlled mode whereas following devices predominantly use the constant-current mode or can be switched into this mode. In comparison to constant-current devices, where the stimulation field is kept stable in size, the stimulation field produced by constant-voltage devices is vulnerable to changing tissue impedances (Lempka et al., 2010; Okun et al., 2012).
The most frequent programming parameters are monopolar stimulation, impulse duration 60–90 μs and frequency 130 Hz (Volkmann et al., 2006). Considering individual symptoms, the amplitude is increased carefully with a simultaneous reduction of dopaminergic medication during several programming sessions. However, the increase of amplitude is limited by stimulation-related side effects such as gait disorder and disequilibrium, dysarthria, oculomotor dysfunction, paraesthesia and increased muscle tone. To decrease these side effects, the stimulation field can be minimized by bipolar stimulation, but the necessity of higher stimulation intensities has to be taken into account (Volkmann et al., 2006). Alternatively, a modification of the stimulation field is achieved by current steering with multiple stimulation sources. Current steering is possible with a new device and allows for an individual shaping of the stimulation field by shifting the current towards another contact (Barbe et al., 2014). Interleaving stimulation is another method to shape the stimulation field by using alternating stimulation of different programs on two electrode contacts. Amongst others, this can be advantageous in patients with motor symptoms that require different contacts or amplitudes for the best therapeutic effect (Wojtecki et al., 2011). Apart from altering the stimulation field, a current pilot study (CUSTOM-DBS) could show that a lower pulse width of 30 μs results in a wider therapeutic spectrum with higher side effect thresholds (Volkmann et al., 2014).
A further stimulation technique under development is directional DBS. In comparison to current devices with omnidirectional stimulation (using one or more contacts of a quadripolar or octopolareelectrode) smaller directional electrodes are used to adapt the stimulated area. Presently available data are based on intraoperative measurements and need to be verified by chronic implantation (Pollo et al., 2014).
Perspective
During the last 25 years, DBS has developed and become an established therapy for PD. Nevertheless, there are ever-growing findings concerning the effectiveness of DBS and the pathomechanisms of side effects resulting in development of new devices and stimulation paradigms. The main aim is a further reduction of side effects and better adaption to individual courses of PD. Inter alia, further development of closed loop stimulation, i.e., adaptive and individual stimulation depending on recorded beta activity of the STN might be an important step for the future (Rosin et al., 2011; Little et al., 2012).
Footnotes
Funding: This study was supported by ERA-NET Neuron /German Federal Ministry of Education and Research (BMBF): TYMON 01EW141 to LW.
References
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–48. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Astrom M, Tripoliti E, Hariz MI, Zrinzo LU, Martinez-Torres I, Limousin P, Wardell K. Patient-specific model-based investigation of speech intelligibility and movement during deep brain stimulation. Stereotact Funct Neurosurg. 2010;88:224–233. doi: 10.1159/000314357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MT, Maarouf M, Alesch F, Timmermann L. Multiple source current steering--a novel deep brain stimulation concept for customized programming in a Parkinson's disease patient. Parkinsonism Relat Disord. 2014;20:471–473. doi: 10.1016/j.parkreldis.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr, Moro E, Vitek JL, Weaver FM, Gross RE, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005;20:224–230. doi: 10.1002/mds.20279. [DOI] [PubMed] [Google Scholar]
- Charles PD, Van Blercom N, Krack P, Lee SL, Xie J, Besson G, Benabid AL, Pollak P. Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology. 2002;59:932–934. doi: 10.1212/wnl.59.6.932. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Rizos A, Sethi KD. Motor and nonmotor complications in Parkinson's disease: an argument for continuous drug delivery? J Neural Transm. 2013;120:1305–1320. doi: 10.1007/s00702-013-0981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano AM, McGrath MM, Willian MK, Snyder CF, LeWitt PA, Reyes PF, Richter RR, Means ED. Evaluation of a measurement strategy for Parkinson's disease: assessing patient health-related quality of life. Qual Life Res. 2000;9:87–100. doi: 10.1023/a:1008928321652. [DOI] [PubMed] [Google Scholar]
- Daniels C, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G, Witt K. Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson's disease. Mov Disord. 2010;25:1583–1589. doi: 10.1002/mds.23078. [DOI] [PubMed] [Google Scholar]
- Demetriades P, Rickards H, Cavanna AE. Impulse control disorders following deep brain stimulation of the subthalamic nucleus in Parkinson's disease: clinical aspects. Parkinsons Dis 2011. 2011 doi: 10.4061/2011/658415. 658415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Dormont D, Seidenwurm D, Galanaud D, Cornu P, Yelnik J, Bardinet E. Neuroimaging and deep brain stimulation. AJNR Am J Neuroradiol. 2010;31:15–23. doi: 10.3174/ajnr.A1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, Albanese A. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain. 2010;133:2664–2676. doi: 10.1093/brain/awq221. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, du Moncel ST, Bonnet AM, Pidoux B, Dormont D, Cornu P, Agid Y. Behavioural disorders, Parkinson's disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72:701–707. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Chang WS, Kang DW, Sohn YH, Lee MS, Chang JW. Factors related to outcomes of subthalamic deep brain stimulation in Parkinson's disease. J Korean Neurosurg Soc. 2013;54:118–124. doi: 10.3340/jkns.2013.54.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, Lang AE, Deuschl G. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- Knobel D, Aybek S, Pollo C, Vingerhoets FJ, Berney A. Rapid resolution of dopamine dysregulation syndrome (DDS) after subthalamic DBS for Parkinson disease (PD): a case report. Cogn Behav Neurol. 2008;21:187–189. doi: 10.1097/WNN.0b013e318185e6e2. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, Ondo W, Pahwa R, Poewe W, Troster AI, Uitti R, Voon V. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(Suppl 14):S171–196. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Gurruchaga JM, Pollin B, von Raison F, Mohsen N, Shin M, Menard-Lefaucheur I, Oshino S, Kishima H, Fenelon G, Remy P, Cesaro P, Gabriel I, Brugieres P, Keravel Y, Nguyen JP. Outcome of bilateral subthalamic nucleus stimulation in the treatment of Parkinson's disease: correlation with intra-operative multi-unit recordings but not with the type of anaesthesia. Eur Neurol. 2008;60:186–199. doi: 10.1159/000148246. [DOI] [PubMed] [Google Scholar]
- Lempka SF, Johnson MD, Miocinovic S, Vitek JL, McIntyre CC. Current-controlled deep brain stimulation reduces in vivo voltage fluctuations observed during voltage-controlled stimulation. Clin Neurophysiol. 2010;121:2128–2133. doi: 10.1016/j.clinph.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, O'sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O'sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson's disease. J Clin Neurosci. 2009;16:1148–1152. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Kühn AA, Brown P. beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236:383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rowe J, Nandi D, Hayward G, Parkin S, Stein J, Aziz T. Localisation of the subthalamic nucleus using Radionics Image Fusion and Stereoplan combined with field potential recording. A technical note. Stereotact Funct Neurosurg. 2001;76:63–73. doi: 10.1159/000056495. [DOI] [PubMed] [Google Scholar]
- Mann JM, Foote KD, Garvan CW, Fernandez HH, Jacobson CEt, Rodriguez RL, Haq IU, Siddiqui MS, Malaty IA, Morishita T, Hass CJ, Okun MS. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. J Neurol Neurosurg Psychiatry. 2009;80:794–797. doi: 10.1136/jnnp.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikos A, Bowers D, Noecker AM, McIntyre CC, Won M, Chaturvedi A, Foote KD, Okun MS. Patient-specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. Neuroimage. 2011;54:S238–246. doi: 10.1016/j.neuroimage.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, Kulisevsky J, Obeso JA, Albanese A, Hariz MI, Quinn NP, Speelman JD, Benabid AL, Fraix V, Mendes A, Welter ML, Houeto JL, Cornu P, Dormont D, Tornqvist AL, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. 2010;25:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- Ondo W, Jankovic J, Schwartz K, Almaguer M, Simpson RK. Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson's disease tremor. Neurology. 1998;51:1063–1069. doi: 10.1212/wnl.51.4.1063. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Meilak C, Vuong KD. Predictors of battery life for the Activa Soletra 7426 Neurostimulator. Parkinsonism Relat Disord. 2007;13:240–242. doi: 10.1016/j.parkreldis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ory-Magne F, Brefel-Courbon C, Simonetta-Moreau M, Fabre N, Lotterie JA, Chaynes P, Berry I, Lazorthes Y, Rascol O. Does ageing influence deep brain stimulation outcomes in Parkinson's disease? Mov Disord. 2007;22:1457–1463. doi: 10.1002/mds.21547. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease. Preliminary experience at Oxford. Br J Neurosurg. 2008;22(Suppl 1):S41–44. doi: 10.1080/02688690802448335. [DOI] [PubMed] [Google Scholar]
- Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, Lozano AM, Raabe A, Schupbach M. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015–2026. doi: 10.1093/brain/awu102. [DOI] [PubMed] [Google Scholar]
- Reck C, Maarouf M, Wojtecki L, Groiss SJ, Florin E, Sturm V, Fink GR, Schnitzler A, Timmermann L. Clinical outcome of subthalamic stimulation in Parkinson's disease is improved by intraoperative multiple trajectories microelectrode recording. J Neurol Surg A Cent Eur Neurosurg. 2012;73:377–386. doi: 10.1055/s-0032-1326957. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Russmann H, Ghika J, Villemure JG, Robert B, Bogousslavsky J, Burkhard PR, Vingerhoets FJ. Subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2004;63:1952–1954. doi: 10.1212/01.wnl.0000144198.26309.d8. [DOI] [PubMed] [Google Scholar]
- Schneider F, Reske M, Finkelmeyer A, Wojtecki L, Timmermann L, Brosig T, Backes V, Amir-Manavi A, Sturm V, Habel U, Schnitzler A. Predicting acute affective symptoms after deep brain stimulation surgery in Parkinson's disease. Stereotact Funct Neurosurg. 2010;88:367–373. doi: 10.1159/000319046. [DOI] [PubMed] [Google Scholar]
- Schüpbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Skodda S, Schlegel U, Südmeyer M, Schnitzler A, Wojtecki L. Effects of levodopa and deep brain stimulation on motor speech performance in Parkinson's disease. Basal Ganglia. 2012;1:49–54. [Google Scholar]
- Skodda S, Gronheit W, Schlegel U, Sudmeyer M, Schnitzler A, Wojtecki L. Effect of subthalamic stimulation on voice and speech in Parkinson's disease: for the better or worse? 2014;4:218. doi: 10.3389/fneur.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeding HM, Goudriaan AE, Foncke EM, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry. 2007;78:517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, Hariz MI, Limousin P. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008;23:2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord. 2006;21(Suppl 14):S284–289. doi: 10.1002/mds.20961. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548–551. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Reich M, Sawalhe AD, Timmermann L, Barbe M, Kühn AA, Hübl J, Schnitzler A, Groiss SJ, Moldovan A, Steinke K, Lin S, Manola L, Carcieri S. 18th International Congress of Parkinson's Disease and Movement Disorders. Late-Breaking Abstracts, MDS Study Group Abstracts and Guided Poster Tour Information. Deep Brain Stimulation at short pulse width results in superior therapeutic windows for treatment of Parkinson's Disease: a randomized, controlled, double-blind neurostimulation trial (CUSTOM-DBS) 12-13, :12-13. 2014. Retrieved from www.mdscongress2014.org/MDS-Files/pdfs/Late-BreakingStudyGroupAbstractsPublication.pdf .
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79:55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Walach M, Meisner C, Fritz M, Scholten M, Breit S, Plewnia C, Bender B, Gharabaghi A, Wachter T, Kruger R. Nigral stimulation for resistant axial motor impairment in Parkinson's disease? A randomized controlled trial. Brain. 2013;136:2098–2108. doi: 10.1093/brain/awt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K. Group PSC (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T, Deuschl G. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain. 2013;136:2109–2119. doi: 10.1093/brain/awt151. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Bötzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Vesper J, Schnitzler A. Interleaving programming of subthalamic deep brain stimulation to reduce side effects with good motor outcome in a patient with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:293–294. doi: 10.1016/j.parkreldis.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Timmermann L, Jörgens S, Südmeyer M, Maarouf M, Treuer H, Gross J, Lehrke R, Koulousakis A, Voges J, Sturm V, Schnitzler A. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Arch Neurol. 2006;63:1273–1276. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]


