Abstract
Electroacupuncture has therapeutic effects on ischemic brain injury, but its mechanism is still poorly understood. In this study, mice were stimulated by electroacupuncture at the Baihui (GV20) acupoint for 30 minutes at 1 mA and 2/15 Hz for 5 consecutive days. A cerebral ischemia model was established by ligating the bilateral common carotid artery for 15 minutes. At 72 hours after injury, neuronal injury in the mouse hippocampus had lessened, and the number of terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-positive cells reduced after electroacupuncture treatment. Moreover, expression of adenosine monophosphate-activated protein kinase α (AMPKα) and phosphorylated AMPKα was up-regulated. Intraperitoneal injection of the AMPK antagonist, compound C, suppressed this phenomenon. Our findings suggest that electroacupuncture preconditioning alleviates ischemic brain injury via AMPK activation.
Keywords: nerve regeneration, electroacupuncture, cerebral ischemia, neuroprotection, adenosine monophosphate-activated protein kinase α, compound C, neurons, apoptosis, NSFC grant, neural regeneration
Introduction
Electroacupuncture (EA) is a special form of acupuncture that has shown therapeutic effects in animal experiments and clinical trials of cerebral ischemic injury (Si et al., 1998). Cerebral ischemic injury tolerance induced by EA preconditioning was first examined in 2003 (Xiong et al., 2003). Moreover, many studies have suggested that Baihui (GV20) acupoint-based acupuncture has a protective effect in the pathophysiological process of ischemic stroke in animal models (Wang et al., 2009, 2011; Kim et al., 2013; Xu et al., 2014b; Zhang et al., 2014; Wu et al., 2015). However, its mechanism is still not well understood, and further studies are required before it becomes clinically accepted.
Expression of adenosine monophosphate-activated protein kinase (AMPK) in adult brain was first reported in 1995. It was suggested that AMPK in the central nervous system plays an important and sophisticated role in energy balance (Gao et al., 1995). The activated form of AMPK contributes to maintaining cellular adenosine triphosphate levels by triggering catabolic processes such as fatty acid oxidation, and inhibiting anabolic pathways including cholesterol synthesis (Ronnett et al., 2009; Li et al., 2015). Previous studies have suggested that the AMPK signaling pathway is involved in cerebral ischemic preconditioning via several mechanisms, alleviating the severe energy deficiency that is often secondary to ischemic brain injury (Culmsee et al., 2001; Ashabi et al., 2014; Jiang et al., 2014, 2015; Jinadasa et al., 2014; Venna et al., 2014).
Numerous studies have demonstrated various EA and AMPK mechanisms during neuroprotection (Tian et al., 2013; Wang et al., 2013; Kim et al., 2014; Viggiano et al., 2014; Xie et al., 2014; Chung et al., 2015). However, the relationship between AMPK and EA is unclear. Therefore, we aimed to determine if the AMPK signaling pathway is involved in neuroprotection induced by EA stimulation targeting the Baihui (GV20) acupoint in a mouse model of bilateral common carotid artery occlusion (BCCAO).
Materials and Methods
Experimental animals
Specific-pathogen-free male C57BL/6 mice (aged 9 weeks old and weighing 20–25 g) were provided by the Cavens experimental animal center (Changzhou, Jiangsu Province, China) (license No. SCXK (Su) 20110003). Experimental mice were bred and housed in the Animal Facility at Qingdao University (China) under controlled conditions in a 12-hour light/dark cycle at 24 ± 2°C with a humidity of 60–70% for at least 1 week before preconditioning or surgery. Mice were allowed free access to a standard rodent diet and tap water. All procedures were approved by the Animal Care and Management Committee of Qingdao University in China (Permit No. QUEC-130205). A total of 60 mice were randomly assigned to five groups (n = 12 in each group): sham, BCCAO, EA + BCCAO, 6-[4-(2-Piperidin-1-yl-ethoxy)phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a]pyrimidine (compound C, CC) + BCCAO, and EA + CC + BCCAO groups.
Electroacupuncture preconditioning
Electroacupuncture preconditioning was performed according to a previously described method (Wang et al., 2005). Briefly, mice were anesthetized intraperitoneally (i.p.) using 4% chloral hydrate (0.1 mL/10 g). The Baihui (GV20) acupoint is located at the intersection of the sagittal midline and the line linking both ears of the rat. The acupoint was subcutaneously acupunctured at 2 mm and stimulated with an intensity of 1 mA and frequency of 2/15 Hz for 30 minutes, once a day, continuously for 5 days using the G6805-1 EA Instrument (XinSheng Co., Ltd., Qingdao, Shandong Province, China). The core temperature of all mice was measured and maintained at 37.0 ± 0.5°C during EA preconditioning by surface heating or cooling.
Establishment of cerebral ischemia model and CC intervention
Mice were subjected to BCCAO for 15 minutes following 5-day eletroacupuncture preconditioning according to a previously described method (Panahian et al., 1996). Briefly, mice were anesthetized using 4% chloral hydrate (i.p., 0.1 mL/10 g). With the neck hyperextended, an anterior midline incision was made through the platysma muscle and fascia propria, and the left and right carotid bundles exposed behind the sternocleidomastoid muscles. The carotid was identified after blunt dissection. Right and left carotid arteries were successively occluded using two Zen temporary clips (13 mm × 0.4 mm; 15 g closing force). Global ischemia was induced for 15 minutes by clip occlusion of both common carotid arteries, followed by 72 hours of reperfusion. Mice in the sham group were subjected to anterior midline incision, but the left and right carotids were only exposed and not clipped.
CC, an AMPK antagonist, was purchased from Merck Millipore (Darmstadt, Germany) and dissolved in dimethyl sulfoxide. At BCCAO onset, CC (10 mg/kg) was injected into the mice (i.p.) (Li et al., 2011). Mice in the sham, BCCAO, and EA + BCCAO groups were injected with normal saline (10 mg/kg).
Terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining and hematoxylin-eosin staining
After 72 hours of reperfusion, cell apoptosis in the hippocampal CA1 region (Paxinos and Watson, 2005) was assessed by hematoxylin-eosin and TUNEL staining. First, the mice (n = 6 in each group) were anesthetized using 4% chloral hydrate (0.1 mL/10 g), and then intracardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Second, the brains were removed and the brain tissue harvested at the coronal plane 1–4 mm posterior to the optic chiasma. Next, the brain tissue was fixed for 2 hours, washed for 4 hours, dehydrated with gradient ethanol, and then cleared with xylene. Lastly, the brain tissue was paraffin-embedded and cut into 4 mm-thick sections for further use. Sections were stained in order with hematoxylin and eosin, dehydrated, and then mounted. All sections were observed by light microscopy. TUNEL staining was performed, as described in a previous study (Liu et al., 2013), and in accordance with the manufacturer's instructions (Roche, Basel, Switzerland). TUNEL staining was quantitatively evaluated using the previously described method. Briefly, sections were examined under a light microscope (Olympus, Tokyo, Japan) at 400× magnification. The total number of positively stained cells within this field-of-view was counted and expressed as cells/mm2. Three animals in each group and three sections for each rat were scored to determine the number of TUNEL-positive cells, with the mean value calculated.
Western blot analysis
After 72 hours of reperfusion, mice (n = 6 in each group) were sacrificed by cervical dislocation under anesthesia using 4% chloral hydrate (i.p., 0.1 mL/10 g). The brains were quickly removed and the hippocampus rapidly isolated and snap-frozen. Hippocampal tissue was homogenized in radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology, Shanghai, China), and centrifuged at 10,000 × g for 5 minutes at 4°C. Protein concentration was measured using the bicinchoninic acid protein assay reagent (Beyotime Biotechnology), and then mixed with buffer and heated at 99°C for 5 minutes. Equal protein amounts (50 μg) were loaded in each well and separated by 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis. Proteins were separately transferred from the gel to polyvinylidinene fluoride membrane (Merck Millipore, Darmstadt, Germany). Membranes were blocked in 5% fat-free milk prepared in Tris-buffered saline/Tween-20 buffer for 1 hour, and incubated with primary monoclonal antibodies for rabbit anti-mouse AMPKα (1:1,000; Abcam, Cambridge, UK), rabbit anti-mouse phosphorylated-AMPKα (p-AMPKα) (1:1,000; Cell Signaling Technology, Danvers, MA, USA), and GAPDH (1:500; rabbit anti-mouse; Zhongshan Goldenbridge Biotechnology, Beijing, China) overnight at 4°C. Membranes were washed three times with Tris-buffered saline/Tween-20, and then incubated with goat anti-rabbit IgG (1:5,000; Zhongshan Goldenbridge Biotechnology) for 2 hours at room temperature. Blots were developed using enhanced chemiluminescence (Beyotime Biotechnology). Semiquantitative analysis of the blots was performed by densitometry with quantification using Quantity One software (Bio-Rad, Hercules, CA, USA). Each sample was immunoblotted three times, and the final optical density value represents the average of these three separate analyses.
Statistical analysis
Data are presented as mean ± SD and were analyzed by one-way analysis of variance followed by the post hoc Student-Newman-Keuls test. All data were analyzed using SPSS 19.0 software for Windows (IBM Corporation, Armonk, NY, USA). A value of P < 0.05 was considered statistically significant.
Results
Effect of EA on hippocampal neuronal morphology in BCCAO mice
Hippocampal CA1 neurons in the sham group appeared normal, and were considered to be indicative of normal neurons (Figure 1). Compared with the sham group, the neuronal morphology in the BCCAO, EA + BCCAO, CC + BCCAO, and EA + CC + BCCAO groups deteriorated to different extents. In comparison with the EA + BCCAO group, more severe neuronal degeneration and disordered neuronal arrangement was observed in the BCCAO, CC + BCCAO, and EA + CC + BCCAO groups after 72 hours of reperfusion.
Figure 1.
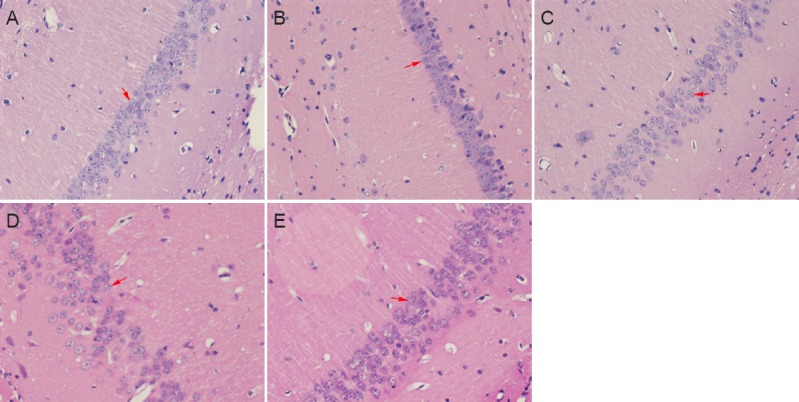
Neuronal morphology in the hippocampal CA1 region at 72 hours after BCCAO (hematoxylin-eosin staining, × 400).
Neurons in the sham group (A) were normal. Neuronal morphology was similar in the EA + BCCAO group (C). Neuronal degeneration was more severe with a more disordered neuronal arrangement in the BCCAO (B), CC + BCCAO (D), and EA + CC + BCCAO (E) groups. Arrows indicate neurons. EA: Electroacupuncture; BCCAO: bilateral common carotid artery occlusion; CC: compound C (an adenosine monophosphate-activated protein kinase antagonist).
Effect of EA on cell apoptosis in the hippocampus of BCCAO mice
Barely any TUNEL-positive cells were detected in the hippocampal CA1 region of mice in the sham group after 72 hours of reperfusion (Figure 2). Compared with the BCCAO group, the number of TUNEL-positive cells in the hippocampal CA1 region decreased in the EA + BCCAO group (P < 0.05). While in contrast to the EA + BCCAO group, the number of TUNEL-positive cells increased in the EA + CC + BCCAO group (P < 0.05).
Figure 2.
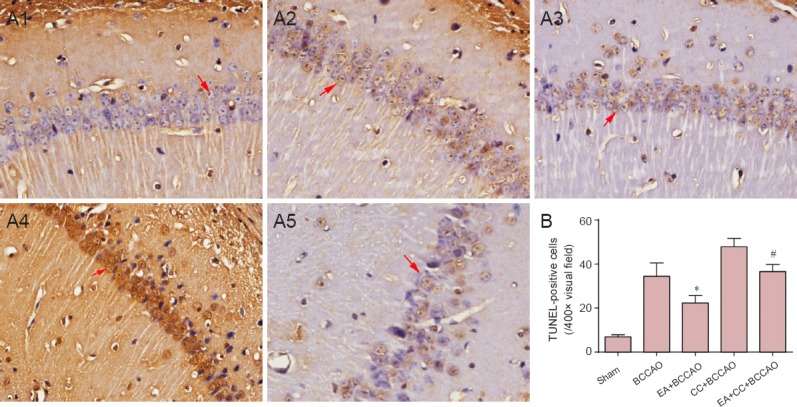
Cell apoptosis at 72 hours after BCCAO in the hippocampal CA1 region (TUNEL staining).
(A) TUNEL staining in the hippocampal CA1 region cells (× 400). (A1–A5) Sham, BCCAO, EA + BCCAO, CC + BCCAO, and EA + CC + BCCAO groups. Arrows indicate typical TUNEL-positive cells. (B) Quantification of TUNEL-positive cells in the hippocmpal CA1 region. Data are presented as the mean ± SD, and were analyzed by one-way analysis of variance followed by post hoc Student-Newman-Keuls test. *P < 0.05, vs. BCCAO group; #P < 0.05, vs. EA + BCCAO group. TUNEL: Terminal deoxynucleotide transferase-mediated dUTP nick-end labeling; EA: electroacupuncture; BCCAO: bilateral common carotid artery occlusion; CC: compound C (an adenosine monophosphate-activated protein kinase antagonist).
Effect of EA on protein expression of AMPKα and p-AMPKα in the hippocampus of BCCAO mice
Expression of AMPKα and p-AMPKα was analyzed by western blot assay (Figure 3). Compared with the sham group, AMPKα and p-AMPKα expression levels in the hippocampal CA1 region were upregulated in the EA + BCCAO group after 72 hours of reperfusion. Compared with the BCCAO group, EA preconditioning significantly upregulated AMPKα and p-AMPKα levels in the EA + BCCAO group (P < 0.05). Moreover, compared with the EA + BCCCAO group, CC intervention had no effect on AMPKα levels in the EA + CC + BCCAO group (P > 0.05), and significantly suppressed increased p-AMPKα expression induced by EA preconditioning in the EA + CC + BCCAO group (P < 0.05).
Figure 3.
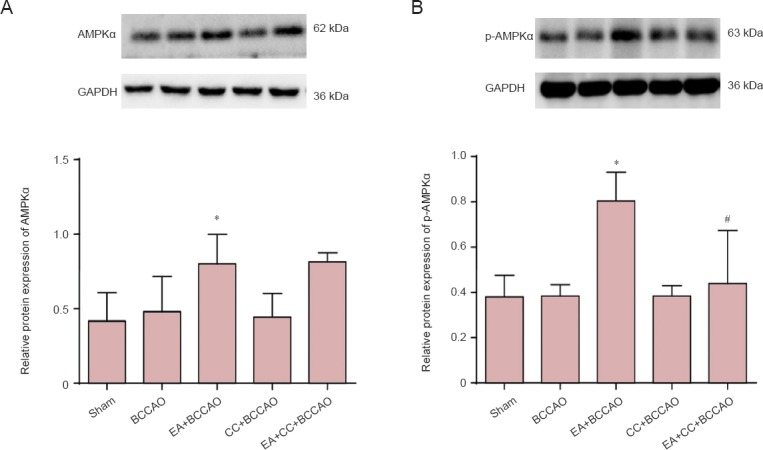
Western blot analysis of protein expression of AMPKα (A) and p-AMPKα (B) in the hippocampal CA1 region at 72 hours after BCCAO.
Data are presented as the mean ± SD (n = 6 mice in each group), and were analyzed by one-way analysis of variance followed by post hoc Student-Newman-Keuls test. Values represent the optical density ratio of target protein to GAPDH. Experiments were performed in triplicate. *P < 0.05, vs. BCCAO group; #P < 0.05, vs. EA + BCCAO group. AMPKα: Adenosine monophosphate-activated protein kinase α; p-AMPKα: phosphorylated adenosine monophosphate-activated protein kinase α; EA: electroacupuncture; BCCAO: bilateral common carotid artery occlusion; CC: compound C (an adenosine monophosphate-activated protein kinase antagonist).
Discussion
As a potent protective maneuver, preconditioning activates several endogenous signaling pathways to provide neuroprotection against ischemia (Pérez-Pinzón et al., 1997; Stagliano et al., 1999; Lee et al., 2015; Parmar and Jones, 2015; Wang et al., 2015). Identification of these pathways and their related targets may lead to development of novel therapeutic concepts (Dirnagl et al., 2009). Experimental studies in animals show that Baihui acupuncture can attenuate ischemic brain injury, and thereby demonstrates the potential value of acupuncture for patients suffering from ischemic brain injury (Gao et al., 2002; Chuang et al., 2007; Cheng et al., 2014; Tan et al., 2014; Xu et al., 2014a). Electroacupuncture based on traditional acupuncture has advantages as it can be readily controlled, standardized, and objectively measured compared with traditional acupuncture.
Recently, AMPK has gained greater interest in the area of neuroprotection. Adenosine monophosphate-activated protein kinase is a heterotrimer with three subunits, including two isoforms of the catalytic α subunit (AMPKα1 and AMPKα2), two isoforms of the β subunit (β1 and β2), and three isoforms of the γ subunit (γ1, γ2, and γ3) (Li and McCullough, 2010). Each subunit appears to have distinct functions and is expressed in different brain areas (Turnley et al., 1999; Culmsee et al., 2001; Ramamurthy and Ronnett, 2006; Hardie, 2007; Ofir et al., 2007; Oakhill et al., 2011). Although AMPK activation is an adaptive response to stress in many systems, the consequences of stress-mediated AMPK activation are still controversial. The role of AMPK has been demonstrated in many studies. During ischemic metabolic stress, AMPK activation is deleterious and its inhibition can lead to neuroprotection (McCullough et al., 2005; Li et al., 2007, 2010; Venna et al., 2012; Nam et al., 2013; Ma et al., 2015). However, other findings show that AMPK represents an endogenous neuroprotective pathway under physiological (hunger) and pathophysiological (stroke) conditions (Culmsee et al., 2001; Kuramoto et al., 2007; Anilkumar et al., 2013; Choi et al., 2013; Jinadasa et al., 2014). It will be important to determine if the role of AMPK varies in different brain regions, and whether its region-specific subunit expression potentially contributes to differing physiological functions.
Expression of AMPKα is particularly high in hippocampal pyramidal neurons, and is activated during energy-deprived states including cerebral ischemia (Pertsch et al., 1988; Hardie, 2004). After ischemia/reperfusion in the hippocampal CA1 region, AMPK immunoreactivity was almost undetectable at 4–7 days, while p-AMPK immunoreactivity was almost undetectable at 1–2 days (Nam et al., 2013). Thus, we chose the time point of 72 hours after ischemia/reperfusion to detect changes in AMPK and p-AMPK levels and obtain a more accurate result. Here, the effect of cerebral ischemia duration on AMPK and p-AMPK expression was ignored, and instead we focused on the influence of EA preconditioning on AMPK and p-AMPK levels. Moreover, the animal model was limited to the C56BL/6 mouse, which is vulnerable to cerebral ischemia. To investigate the effects of EA preconditioning, CC was used to inhibit AMPK activation. CC is a selective pharmacological AMPK inhibitor and the only available agent that has been widely used in cell-based, biochemical, and in vivo assays (Vucicevic et al., 2011). CC can reduce AMPK phosphorylation and enzymatic activity, which results in diminished functional AMPK (Vucicevic et al., 2009).
First, hematoxylin-eosin and TUNEL staining revealed that EA preconditioning suppressed cell apoptosis, confirming the protective effect of EA in the BCCAO model. Second, western blot analysis showed that AMPKα protein levels were increased by EA preconditioning in the hippocampal CA1 region after 72 hours of reperfusion in the EA + BCCAO and EA + CC + BCCAO groups. However, because of AMPK inactivation, TUNEL staining revealed a significant difference in cell apoptosis between these two groups. Lastly, p-AMPKα expression and the p-AMPKα/AMPKα ratio in the EA + CC + BCCAO group increased more significantly than in the other groups. Western blot analysis showed that p-AMPKα expression and the p-AMPKα/AMPKα ratio in the EA + CC + BCCAO group were lower compared with the EA + BCCAO group. TUNEL staining suggested that cell apoptosis is greater in the EA + CC + BCCAO group, indicating that AMPK-induced neuroprotection is inhibited by CC.
Our study shows that EA preconditioning significantly reduces neuronal apoptosis, whereas CC after EA stimulus reverses the beneficial effect, suggesting that EA preconditioning alleviates neuronal apoptosis via AMPK activation. Thus, this study has further delineated the protective effect of EA preconditioning on ischemic brain injury. Although further investigations are needed to identify the detailed signaling cascades underlining the AMPK pathway in EA pretreatment, our current findings highlight a novel mechanism for EA pretreatment inducing rapid tolerance to cerebral ischemia in a mouse model.
Acknowledgments
We are very grateful to Dr. Bing Luo from Qingdao University in China for generously providing the laboratory.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81273821.
Conflicts of interest: None declared.
Copyedited by James R, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Anilkumar U, Weisová P, Düssmann H, Concannon CG, König HG, Prehn JH. AMP-activated protein kinase (AMPK)-induced preconditioning in primary cortical neurons involves activation of MCL-1. J Neurochem. 2013;124:721–734. doi: 10.1111/jnc.12108. [DOI] [PubMed] [Google Scholar]
- Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab Brain Dis. 2014;29:47–58. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Lin JG, Tang NY, Kao ST, Hsieh CL. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS One. 2014;9:e91426. doi: 10.1371/journal.pone.0091426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Ju C, Anthony Jalin AM, Lee da I, Prather PL, Kim WK. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol. 2013;182:928–939. doi: 10.1016/j.ajpath.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Chuang CM, Hsieh CL, Li TC, Lin JG. Acupuncture stimulation at Baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injured sprague-dawley rats. Am J Chinese Med. 2007;35:779–791. doi: 10.1142/S0192415X07005260. [DOI] [PubMed] [Google Scholar]
- Chung MM, Chen YL, Pei D, Cheng YC, Sun B, Nicol CJ, Yen CH, Chen HM, Liang YJ, Chiang MC. The neuroprotective role of metformin in advanced glycation end product treated human neural stem cells is AMPK-dependent. Biochim Biophys Acta. 2015;1852:720–731. doi: 10.1016/j.bbadis.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp B, Mattson M. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Widmer J, Stapleton D, Teh T, Cox T, Kemp BE, Witters LA. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta. 1995;1266:73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Gao H, Guo J, Zhao P, Cheng J. The neuroprotective effects of electroacupuncture on focal cerebral ischemia in monkey. Acupunct Electrother Res. 2002;27:45–57. doi: 10.3727/036012902816026112. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway – new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146–3157. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Shi JQ, Gao L, Qin H, Zhang YD, Tan L. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol Neurobiol. 2015;51:220–229. doi: 10.1007/s12035-014-8725-6. [DOI] [PubMed] [Google Scholar]
- Jinadasa T, Szabó EZ, Numata M, Orlowski J. Activation of AMP-activated protein kinase regulates hippocampal neuronal pH by recruiting Na(+)/H(+) exchanger NHE5 to the cell surface. J Biol Chem. 2014;289:20879–20897. doi: 10.1074/jbc.M114.555284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi KH, Jang YJ, Kim HN, Bae SS, Choi BT, Shin HK. Electroacupuncture preconditioning reduces cerebral ischemic injury via BDNF and SDF-1α in mice. BMC Complement Altern Med. 2013;13:22–22. doi: 10.1186/1472-6882-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Kim HN, Ahn SM, Choi YH, Shin HK, Choi BT. Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia. PLoS One. 2014;9:e90000. doi: 10.1371/journal.pone.0090000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Warren N, Tamaki K, Iemata M, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Cho JH, Kim IH, Ahn JH, Park JH, Cho GS, Chen BH, Shin BN, Tae HJ, Park SM, Ahn JY, Kim DW, Cho JH, Bae EJ, Yong JH, Kim YM, Won MH, Lee YL. Ischemic preconditioning inhibits expression of Na+/H+ exchanger 1 (NHE1) in the gerbil hippocampal CA1 region after transient forebrain ischemia. J Neurol Sci. 2015;351:146–153. doi: 10.1016/j.jns.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Benashski S, McCullough LD. Post-stroke hypothermia provides neuroprotection through inhibition of AMP-activated protein kinase. J Neurotrauma. 2011;28:1281–1288. doi: 10.1089/neu.2011.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Benashski SE, Siegel C, Liu F, McCullough LD. Adenosine monophosphate activated protein kinase inhibition is protective in both sexes after experimental stroke. Neurosci Lett. 2010;482:62–65. doi: 10.1016/j.neulet.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6:7365–7378. doi: 10.18632/oncotarget.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang M, Chen H, Guo Y, Ma F, Shi F, Bi Y, Li Y. Hypothermia protects the brain from transient global ischemia/reperfusion by attenuating endoplasmic reticulum response-induced apoptosis through CHOP. PLoS One. 2013;8:e53431. doi: 10.1371/journal.pone.0053431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ma Y, Bu J, Dang H, Sha J, Jing Y, Shan-jiang AI, Li H, Zhu Y. Inhibition of adenosine monophosphate-activated protein kinase reduces glial cell-mediated inflammation and induces the expression of Cx43 in astroglias after cerebral ischemia. Brain Res. 2015;1605:1–11. doi: 10.1016/j.brainres.2014.11.030. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Nam HG, Kim W, Yoo DY, Choi JH, Won MH, Hwang IK, Jeong JH, Hwang HS, Moon SM. Chronological changes and effects of AMP-activated kinase in the hippocampal CA1 region after transient forebrain ischemia in gerbils. Neurol Res. 2013;35:395–405. doi: 10.1179/1743132813Y.0000000158. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- Ofir M, Hochhauser E, Vidne BA, Freimark D, Arad M. AMP-activated protein kinase: how a mistake in energy gauge causes glycogen storage. Harefuah. 2007;146:770–775, 813-814. [PubMed] [Google Scholar]
- Pérez-Pinzón MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Panahian N, Yoshida T, Huang PL, Hedley-Whyte ET, Dalkara T, Fishman MC, Moskowitz MA. Attenuated hippocampal damage after global cerebral ischemia in mice mutant in neuronal nitric oxide synthase. Neuroscience. 1996;72:343–354. doi: 10.1016/0306-4522(95)00563-3. [DOI] [PubMed] [Google Scholar]
- Parmar J, Jones NM. Hypoxic preconditioning can reduce injury-induced inflammatory processes in the neonatal rat brain. Int J Dev Neurosci. 2015;43:35–42. doi: 10.1016/j.ijdevneu.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pertsch M, Duncan GE, Stumpf WE, Pilgrim C. A histochemical study of the regional distribution in the rat brain of enzymatic activity hydrolyzing glucose- and 2-deoxyglucose-6-phosphate. Histochemistry. 1988;88:257–262. doi: 10.1007/BF00570281. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si QM, Wu GC, Cao XD. Effects of electroacupuncture on acute cerebral infarction. Acupunct Electrother Res. 1998;23:117–124. doi: 10.3727/036012998816356562. [DOI] [PubMed] [Google Scholar]
- Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Tan F, Chen J, Liang Y, Gu M, Li Y, Wang X, Meng D. Electroacupuncture attenuates cervical spinal cord injury following cerebral ischemia/reperfusion in stroke-prone renovascular hypertensive rats. Exp Ther Med. 2014;7:1529–1534. doi: 10.3892/etm.2014.1619. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tian WQ, Peng YG, Cui SY, Yao FZ, Li BG. Effects of electroacupuncture of different intensities on energy metabolism of mitochondria of brain cells in rats with cerebral ischemia-reperfusion injury. Chin J Integr Med. 2013 doi: 10.1007/s11655-013-1512-9. doi: 10.1007/s11655-013-1512-9. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- Venna VR, Li J, Benashski SE, Tarabishy S, McCullough LD. Preconditioning induces sustained neuroprotection by downregulation of adenosine 5′-monophosphate-activated protein kinase. Neuroscience. 2012;201:280–287. doi: 10.1016/j.neuroscience.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci. 2014;39:2129–2138. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano E, Viggiano D, Viggiano A, De Luca B, Monda M. Cortical spreading depression increases the phosphorylation of AMP-activated protein kinase in the cerebral cortex. Neurochem Res. 2014;39:2431–2439. doi: 10.1007/s11064-014-1447-3. [DOI] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E, Sumarac-Dumanovic M, Micic D, Trajkovic V. AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684–1693. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic, Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- Wang F, Gao Z, Li X, Li Y, Li X, Zhong H, Xu N, Cao F, Wang Q, Xiong L. NDRG2 is involved in anti-apoptosis induced by electroacupuncture pretreatment after focal cerebral ischemia in rats. Neurol Res. 2013;35:406–414. doi: 10.1179/1743132813Y.0000000159. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xiong L, Chen S, Liu Y, Zhu X. Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett. 2005;381:158–162. doi: 10.1016/j.neulet.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40:2157–2164. doi: 10.1161/STROKEAHA.108.541490. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li X, Chen Y, Wang F, Yang Q, Chen S, Min Y, Li X, Xiong L. Activation of epsilon protein kinase C-mediated anti-apoptosis is involved in rapid tolerance induced by electroacupuncture pretreatment through cannabinoid receptor type 1. Stroke. 2011;42:389–396. doi: 10.1161/STROKEAHA.110.597336. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu C, Li S, Xu Y, Chen P, Liu Y, Ding Q, Wahafu W, Hong B, Yang M. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One. 2015;10:e0118951. doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zou Z, Zou R, Zhou X, Cui S. Electroacupuncture pretreatment induces tolerance against cerebral ischemia/reperfusion injury through inhibition of the autophagy pathway. Mol Med Rep. 2015;11:4438–4446. doi: 10.3892/mmr.2015.3253. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ding SQ, Shen YF. Silibinin activates AMP-activated protein kinase to protect neuronal cells from oxygen and glucose deprivation-re-oxygenation. Biochem Biophys Res Commun. 2014;454:313–319. doi: 10.1016/j.bbrc.2014.10.080. [DOI] [PubMed] [Google Scholar]
- Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang Q, Chen S. Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin Med J (Engl) 2003;116:108–111. [PubMed] [Google Scholar]
- Xu H, Zhang Y, Sun H, Chen S, Wang F. Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS One. 2014a;9:e97488. doi: 10.1371/journal.pone.0097488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Li W, Liang Y, Yang Z, Liu J, Wang Y, Su N. Neuroprotective effects of electro acupuncture on hypoxic-ischemic encephalopathy in newborn rats Ass. Pak J Pharm Sci. 2014b;27:1991–2000. [PubMed] [Google Scholar]
- Zhang YM, Xu H, Sun H, Chen SH, Wang FM. Electroacupuncture treatment improves neurological function associated with regulation of tight junction proteins in rats with cerebral ischemia reperfusion injury. Evid Based Complement Alternat Med 2014. 2014 doi: 10.1155/2014/989340. 989340. [DOI] [PMC free article] [PubMed] [Google Scholar]


