Abstract
Human ageing affects the immune system resulting in an overall decline in immunocompetence. Although all immune cells are affected during aging, the functional capacity of T cells is most influenced and is linked to decreased responsiveness to infections and impaired differentiation. We studied age-related changes in DNA methylation and gene expression in CD4+ and CD8+ T cells from younger and older individuals. We observed marked difference between T cell subsets, with increased number of methylation changes and higher methylome variation in CD8+ T cells with age. The majority of age-related hypermethylated sites were located at CpG islands of silent genes and enriched for repressive histone marks. Specifically, in CD8+ T cell subset we identified strong inverse correlation between methylation and expression levels in genes associated with T cell mediated immune response (LGALS1, IFNG, CCL5, GZMH, CCR7, CD27 and CD248) and differentiation (SATB1, TCF7, BCL11B and RUNX3). Our results thus suggest the link between age-related epigenetic changes and impaired T cell function.
Ageing progressively affects the activity of the immune system. The age-related decline in immune responses, also termed immunosenescence, is prominent in the adaptive immune system, where T cells appear to be most affected during ageing1,2. Both CD4+ and CD8+ T cell compartments change with age, however, the CD8+ T cell population in particular becomes enriched for a high proportion of terminally differentiated effector cells, which have lower responsiveness to antigens1. Chronic infection with cytomegalovirus (CMV) is implicated in the age-dependent expansion of the CD8+ T cell subset3,4, and in the older adults, 25–50% of the CD8+ cells can be specific to CMV5,6,7. A typical feature of ageing is a chronic, low-grade inflammatory status, named inflamm-ageing, which is characterized by a general increase in proinflammatory cytokines8,9. The increased expression of the proinflammatory markers suggests that immunosenescence is driven by a chronic antigenic load, which induces the proinflammatory phenotype10. Accordingly, the concept of an immune risk profile, associated with higher mortality rates, has been proposed. This is composed of depleted naïve T cell numbers, an inverted CD8+/CD4+ T cell ratio, low responses to mitogen stimulation, increased population of anergic terminally differentiated CD8+ T cells and increased pro-inflammatory cytokine expression11.
Age-related transcriptome marker analyses in peripheral blood leukocytes (PBL) or mononuclear cells have revealed changes in gene expression levels and aberrant mRNA splicing12,13,14, with gender-specific effects15 and dependence upon CMV phenotype16. Several studies have used peripheral blood cells for the analysis of DNA methylation changes during ageing, and have revealed differentially methylated CpG sites17,18, hypermethylation of CpG islands in promoters19,20, existence of mega-base scale hypomethylated blocks21, and proposed methylation biomarkers as predictors of age22,23,24. However, it should be noted that the transcriptional and epigenetic analyses in human peripheral blood samples are affected by the numerical alteration of specific cell subsets in blood including T cells, B cells, monocytes and granulocytes25,26. Even if normalization tools are applied27, the use of purified cell subsets is superior to the identification of methylome changes and their correlation to cell-type specific functions.
In light of these considerations we focused on sorted CD4+ and CD8+ T cells and aimed to identify whether the DNA methylation changes are involved in development of age-related decrease of T cell responsiveness. We purified DNA from PBL, CD4+ and CD8+ T cells from younger and older individuals, and compared their genome-wide DNA methylation differences. We correlated the age-related methylation changes with gene expression levels and histone modifications in the same regions. Our genome-wide analyses indicate for the first time in CD8+ T cell subset a strong age-related correlation of DNA methylation and the expression of genes having functional role in controlling T cell immune responses and their differentiation.
Results
Age-related methylation changes in PBL, CD4+ and CD8+ T cells
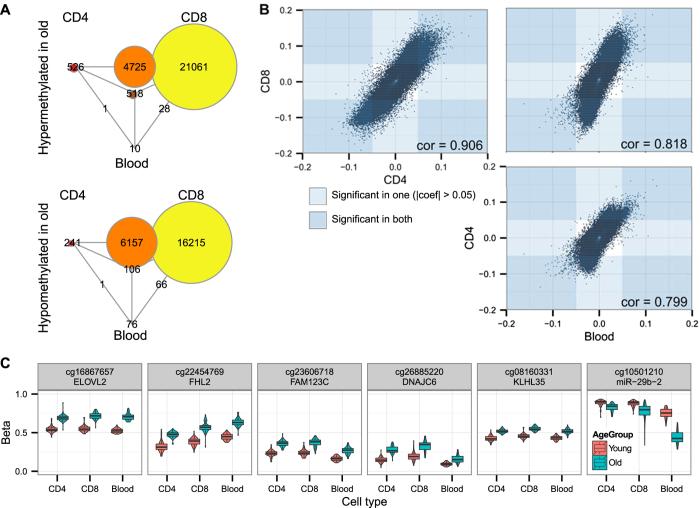
We studied the methylomes in PBL, and CD4+ and CD8+ T cells from 50 younger (age 22–34) and 50 older (age 73–84) individuals using the Illumina HumanMethylation450 arrays. For the differential methylation analysis, we used a linear model to remove confounding effects (see Materials and Methods for details). As the numbers of leukocyte subsets in blood vary greatly with age and skew the analysis of PBL samples, we applied additional normalization based on cell numbers in PBL samples to remove the effect of variable numbers of granulocyte, lymphocyte and monocyte subsets in each PBL sample based on measured and estimated hematological cell counts. After the normalization for variation in cell counts, we confirmed only a small proportion of the differentially methylated sites in PBL (7725 before and 806 after the normalization). In contrast, we identified 12,275 and 48,876 differentially methylated CpG sites in the purified CD4+ and CD8+ T cells (Fig. 1A). The full list of methylation changes is accessible via Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE59065). The overall direction (hyper- or hypomethylation) of age-related methylation changes in CD4+ and CD8+ T cells was in strong correlation (r > 0.9) (Fig. 1B). Less than 2% of the age-related methylation sites in CD4+ and CD8+ T cells could be detected in PBLs, and majority of them were hypermethylated in older population. Nevertheless, all top sites with the highest methylation differences between younger and older individuals belonged to this group shared by CD4+, CD8+ T cells and PBLs (Fig. 1C).
Figure 1. Differentially methylated sites in PBL, CD4+ and CD8+ T cells.
(A) Venn diagram showing the overlaps between age-related differentially methylated sites in PBL, CD4+ and CD8+ T cells. The circles in the corners show the number of CpG sites unique to each cell type and the intermediate circles show the number of overlapping sites between the respective cell types. (B) Correlation of fold changes for all sites. The regions of the plot that correspond to |fold change| > 0.05 are indicated by blue rectangles (t-test adjusted p-value < 0.01). (C) Methylation levels of the top differentially methylated CpG sites in CD4+ and CD8+ T cells and in PBL samples.
Increased methylome variation in aged CD8+ T cells
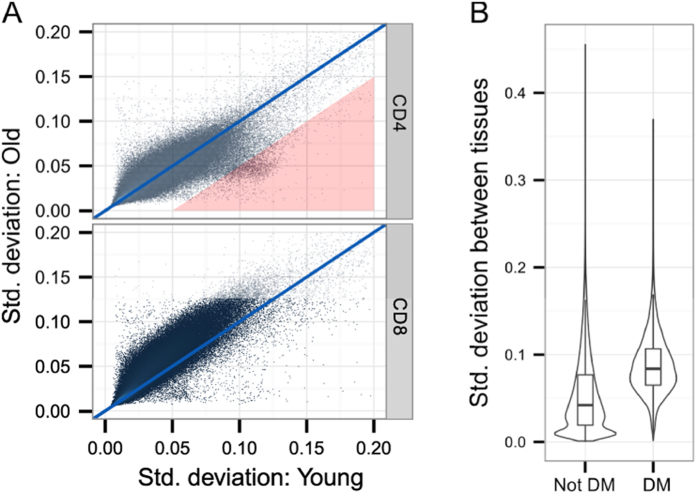
The average methylation levels between CD4+ and CD8+ T cells from younger and older individuals were similar across different genomic locations (Supplementary Fig. S1A). However, given the high number of methylation changes and expansion of terminally differentiated CD8+ T cells with age, we expected the CD8+ T cell methylome to be more variable among the old age group. Indeed, we found increased age-related variability among majority of CpG sites in CD8+ T cells (Fig. 2A; note shift in methylome variability of CD8+ T cells from older individuals). This increased variation of CpG methylation in CD8+ T cells from older individuals was present in all gene and CpG island subregions (Supplementary Fig. S1B). On average, the CpG methylation variation levels in CD4+ T cells changed less with age, with the exception of a subset of CpG sites enriched for genes involved in wound healing according to Gene Ontology (GO) analysis (Fig. 2A, Supplementary Table S1). We then tested whether the CpG sites that were differentially methylated in CD8+ T cells of older individuals are in general more variable in somatic cells and compared our results with the methylomes from 17 human somatic tissues28. We found that the differentially methylated CpG sites had also higher variability in other tissues (Fig. 2B). Together the results showed that CD8+ T cells had higher age-related methylation variation than CD4+ T cells and that the majority of age-related changes in CD8+ T cells occur at CpG sites that are variably methylated in multiple tissues.
Figure 2. Methylation variability in T cells.
(A) The scatter plots compare the standard deviation of every site within younger (x-axis) and older (y-axis) individuals. In CD4+ T cells (upper panel) there is a small subset of sites (marked with a red triangle) that is more variable among young subjects. This subset is enriched in genes involved in hemostasis according to g:Profiler Gene Ontology search (see Supplementary Table S2 for details). In CD8+ T cells (lower panel) majority of sites are more variable in older individuals. (B) Box/violin plots showing the variability of the differentially methylated (DM) and other sites (Not DM) in comparison with the methylation variability in a compendium of 17 different tissues. Note higher standard deviation value among differentially methylated sites.
Age-related hypermethylation occurs at CpG islands and regions with repressive histone marks
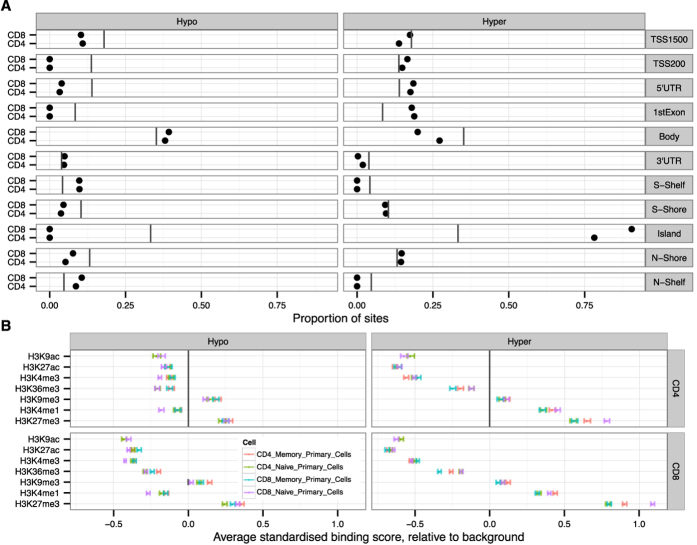
We found a large proportion (>80%) of the hypermethylated CpG sites located in CpG islands, while the hypomethylated CpG sites were more located at the borders of the CpG islands (CpG island shelves) and in gene bodies in both CD4+ and CD8+ T cells (Fig. 3A). Hypermethylation was also increased at gene regulatory (TSS200, 5´ UTR and 1st exon) regions whereas hypomethylation was preferentially decreased in gene regulatory regions. This suggested that the hypermethylated regions are enriched in CpG islands located at 5´gene regulatory regions. We then analysed the histone modifications around the differentially methylated sites. To this end, we overlapped the differentially methylated sites in T cells with the reported locations of seven histone modifications in naïve and memory CD4+ and CD8+ T cells, available from the Roadmap Epigenomics project (Fig. 3B). The comparison showed similar distribution of histone marks in naïve and memory subsets of CD4+ and CD8+ T cells. Both age-related hypo- and hypermethylated regions were enriched in repressed chromatin marks (H3K27me3 and H3K9me3). In agreement, the differentially methylated sites had also lower levels of histone marks associated with active promoters (H3K9ac and H3K4me3), enhancers (H3K27ac) or exon regions (H3K36me3). However, another enhancer-associated modification, H3K4me1, which has been also associated with transposable elements29, was abundant at hypermethylated regions in older individuals. In summary, these results showed the enrichment of age-related hypermethylation at CpG islands and in regions with repressive histone marks.
Figure 3. Location of differentially methylated sites in relation to gene subregions and histone modifications.
(A) The proportions of differentially hypo- and hypermethylated sites that are related to various gene and CpG island subregions are marked with black dots. The vertical lines show the proportion of all CpG sites of HumanMethylation 450 K array located within each subregion. (B) The epigenetic landscape near the differentially methylated sites using Roadmap Epigenomics histone modification data from naïve and memory CD4+ and CD8+ cells. The figure shows the average difference in standardized binding score between differentially methylated and background of non-differentially methylated sites. The influence of gene and island subregion distribution for the sites (shown in Panel A) was subtracted before the analysis.
Age-related differential methylation occurs at repressed genes but in a subset of genes correlates inversely with expression
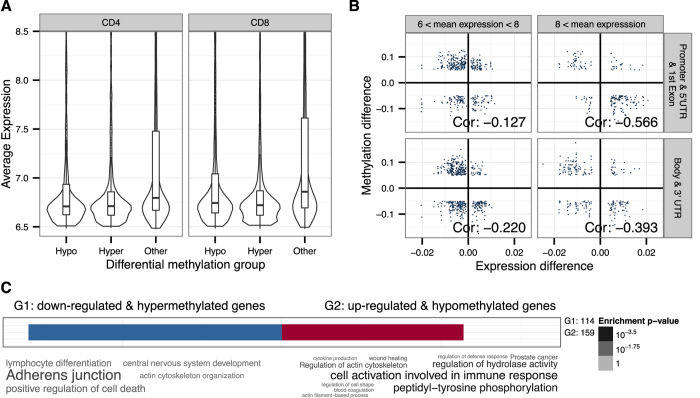
To show that the age-related CpG methylation occurs near transcriptionally repressed genes, we analysed the expression profiles of the CD4+ and CD8+ T cells using the HumanHT-12 v4 Expression array. We compared the differentially methylated sites in respect to the average expression level of their nearest gene. Figure 4A shows that irrespective of the direction of methylation change, the differentially methylated sites were preferentially associated with genes having low or no expression.
Figure 4. Gene expression and its relation to differential methylation.
(A) The average log2 transformed expression levels of genes in CD4+ and CD8+ T cells are compared among the genes with decreased (Hypo), increased (Hyper) and unchanged (Other) methylation during ageing. The gene expression values lower than 6.5 are not included as they correspond to non-detectable gene expression. (B) The correlation between differentially expressed genes (x-axis) and corresponding differentially methylated sites (y-axis) in CD8+ T cells. Each dot corresponds to a CpG site-gene pair. The subplots are defined based on the average expression level and the location of the CpG site. (C) Functional annotation of genes (from panel B) with inverse correlation between their expression and the methylation of nearby CpG sites as word clouds. G1 corresponds to the group of genes with downregulated expression and hypermethylation and G2 corresponds to the group of genes with upregulated expression and hypomethylation (see Supplementary Table S2 for details).
We then studied the correlation of DNA methylation and expression in CD4+ and CD8+ T cells (Supplementary Table S2). Importantly, in a subset of genes that were expressed in CD8+ T cells (>log 6), we observed an inverse correlation between gene expression and DNA methylation (Fig. 4B). The inverse correlation was stronger among methylation sites that were located in gene regulatory and promoter regions and among genes with higher expression levels (>log 8). Functional annotation of the genes with the inverse correlation revealed their association with cell activation involved in immune response, tyrosine phosphorylation and lymphocyte differentiation among the hypomethylated genes (Fig. 4C, Supplementary Table S3). We then searched the promoter regions of the genes with inverse correlation of methylation and expression in CD8+ T cells for binding sites of transcription factors, which could be involved in the recruitment of DNA methylation modifier enzymes. We found a significant overrepresentation of sites for C2H2 zinc finger family transcription factors KLF4, MZF1 and SP1 (Supplementary Table S3), regardless of the expression levels of the genes. Together, the expression analysis confirmed that the majority of the differentially methylated sites are located near genes with low transcriptional activity. However, among the genes that were differentially expressed during ageing, we found significant inverse correlation between methylation and gene expression.
DNA methylation correlates with the expression of genes involved in immune response and lineage differentiation
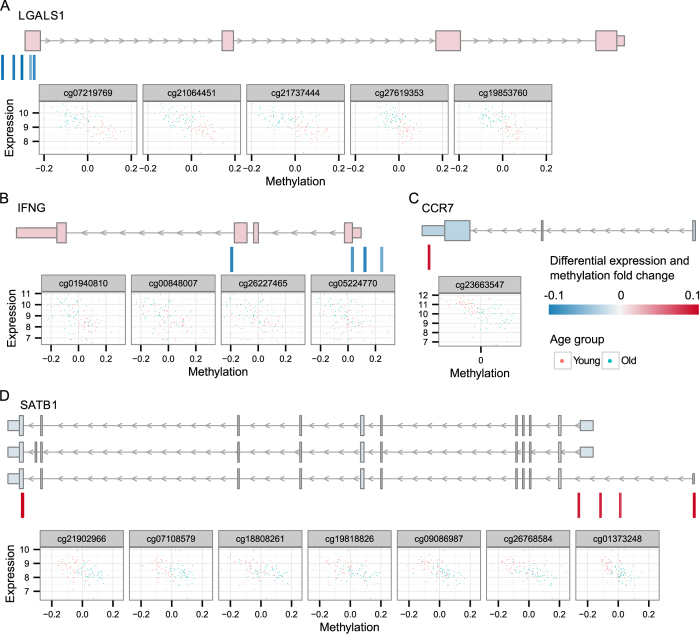
To assess the potential functional effect of DNA methylation changes in aged T cells, we focused on the genes with inverse correlation between methylation and expression: 10 genes in CD4+ and 272 genes in CD8+ T cells (Supplementary Table S2). We found strong inverse correlation with the LGALS1 gene encoding galectin 1, which is known to have a strong suppressive effect on T cell mediated immune responses due to its activity to induce apoptosis of activated T cells30. The increased expression of LGALS1 with decreased methylation at its promoter region was present in both aged CD8+ and CD4+ T cells (Fig. 5). The other known genes with decreased methylation and increased expression in aged CD8+ T cells were the proinflammatory mediators IFNG and CCL5, as well as the cytolysis enzyme granzyme GZMH involved in CD8+ T cells effector functions (Supplementary Fig. S2). By contrast, older individuals showed increased methylation and decreased expression of the chemokine receptor CCR7 responsible for T cell homing to lymph nodes and activation31, the membrane surface marker CD27 involved in T cell expansion and induction of long-term memory32,33 and CD248 which regulates the proliferation of T cells34. Furthermore, we observed negative correlation for several master transcriptional regulators of the T cell lineage such as SATB1, a chromatin organizer in T cells, TCF7, a T cell specific factor that induces the T cell differentiation program and controls the expression of the CD3E molecule, as well as BCL11B and RUNX3, the crucial regulators of the T cell lineage. Among other top genes with inverse correlation between methylation and gene expression we found RGS10, a regulator of G-protein signalling acting as an inhibitor of T cell adhesion and KLRD1/CD94, a receptor for the HLA-E molecules. To confirm our array results, we verified the correlations for several genes (IFNG, GZMH, CCR7, CD27, CD248, and SATB1) using the Sequenom MassARRAY and real-time quantitative PCR in a smaller subset of samples from younger and older persons (Supplementary Fig. S3).
Figure 5. Examples of genes with known functional role in CD8+ T cells that display inverse correlation between gene expression and DNA methylation.
(A) LGALS1, (B) IFNG, (C) CCR7 and (D) SATB1 gene. Each panel is composed of three sections. The top sections display the different transcripts of the gene, marked according to the expression level fold change between younger and older individuals. The middle sections show the associated CpG sites that are differentially methylated. The scatter plots in the bottom sections illustrate the correspondence between expression and methylation levels for each site separately. The scatter plots are displayed in the same order as the sites in the middle section; the red and blue dots display levels detected in the younger and older individuals, respectively.
Discussion
We here report age-related methylation changes that were identified in CD4+ and CD8+ T cells by analyzing more than 400,000 CpG sites from a total of 100 individuals.
The tissue-specificity of age-related DNA methylation has been reported previously35, yet several genome-wide studies have demonstrated similar, but not identical, age-related methylation changes in different tissues36,37,38,39. When we applied normalization based on measured granulocyte, monocyte and lymphocyte counts in our donor individuals to the blood DNA methylation profiles, the number of differential methylation sites in PBL samples decreased remarkably, indicating that the blood cell proportions that differ between individuals have impact on overall analysis of DNA methylation. However, the most significant methylation changes remained - they were shared by PBL and T cell samples and cannot be explained by the variation of blood cell proportions. This finding is in agreement with the recent study showing that most prominent epigenetic changes identified in blood cells retain their significance even after the analysis is adjusted for shifts in blood cell subtypes21. The top age-related DNA methylation changes found in any leukocyte subclass could accumulate in precursor cells at earlier stages of differentiation, for example during haematopoiesis, or reflect more general phenomenon of epigenetic drift with time. Nevertheless, our data show that although both CD4+ and CD8+ T cells share age-related methylation changes with PBL, many additional DNA methylation changes specific to T cells occur with age.
It should be noted that thymic involution influences T cell population during ageing40. T cells from older persons tend to have decreased percentages of naive cells and increased proportion of memory cells, which in CD8+ T cell compartment accumulate as terminally differentiated effector memory cells41. One of the limitations of our study is that the proportions of the naïve and memory cells differ between young and old people, and this could partly explain some of the methylation changes seen in our analysis. In agreement with the oligoclonal expansion and accumulation of terminally differentiated CD8+ cells, we found higher number of DNA methylation changes and increased methylation variation in aged CD8+ T cells in comparison to CD4+ cells. Whether the increased differential methylation is associated with the proliferation of CD8+ T cells in response to chronic CMV infection needs further studies.
Majority of the hypermethylated CpG sites were located in CpG islands, at silent gene promoter regions and were enriched for repressive marks such as H3K27me3, confirming the earlier reported links between age-related hypermethylation, gene inactivation and chromatin condensation42. Indeed, majority of age-related methylation changes seem not to affect the expression of nearby-positioned genes. However, in a subset of genes expressed in CD8+ T cells, we found a negative correlation between DNA methylation and transcription levels. Among those we identified genes with critical roles in T cell mediated immune responses. We found decreased methylation and increased expression of galectin 1 gene (LGALS1) that has multiple functions in suppressing immune responses, including controlling of T cell survival, TCR mediated signalling, regulatory T cell function, and by inhibiting anti-tumor T cell responses30,43,44. In addition, we found in older people the hypomethylation at the IFNG promoter, which correlated with the higher expression of the gene in their CD8+ T cells. Previous studies have shown high production of IFNγ the major proinflammatory cytokine, by activated CD8+ T cells after the stimulation by CMV antigens10,45. Decreased levels of DNA methylation and H3K4me3 repressive marks have been found at Ifng gene after the activation and differentiation of mouse CD8+ T cells in response to infections46. We also found hypomethylation and increased expression of the proinflammatory chemokine CCL5, the plasma levels of which are known to increase with age10, and of the GZMH gene, which is upregulated in effector T cells during infections and in chronic inflammatory diseases47. Demethylation of the Ifng, Ccl5 and granzyme genes occurs during viral infection-induced differentiation of mouse effector and memory CD8+ T cells48,49. Furthermore, our finding of promoter hypermethylation of costimulatory CD27 and chemokine CCR7 receptor genes is in agreement with their downregulation in terminally differentiated anergic CD8+ T cells observed in aged individuals41. In this light, it is tempting to speculate that age-related chronic viral infections, such as CMV, may induce extensive oligoclonal proliferation of CD8+ T cells and result in changed DNA methylation profiles at genes involved in T cell responses to viral infections and in chronic inflammation.
T cell differentiation programme to specialized effector cells is guided by the action of several distinct transcription regulators. In CD8+ T cells, we identified age-related hypermethylation at several transcriptional regulator genes required for T cell lineage differentiation. SATB1, the T lineage-enriched global chromatin organizer, has important roles in T cell development and proliferation and ensures proper development of the lineage50,51. Furthermore, three other genes regulating T cell differentiation, TCF7, BCL11B and RUNX352,53,54,55 were hypermethylated and had lower expression level in CD8+ T cells from older individuals. The expression of TCF7, BCL11B and RUNX3 genes is required for the mature CD8+ T cell differentiation and is decreased with the acquisition of effector cell phenotype56,57. Our data thus show that ageing is associated with decreased expression and DNA hypermethylation of central T cell specific transcriptional regulator genes with fundamental roles in CD8+ T cell differentiation. Together, our results support the concept that the silencing of transcriptional regulator genes by DNA hypermethylation during ageing directs the gene expression profile towards the terminally differentiated effector CD8+ T cells.
In conclusion, although epigenome-wide studies with PBL have identified genes with methylation changes associated with age, the purification of cell subtypes allows more precise investigation of changes relevant to the altered function of specific cells. Our study shows that most of the gains in methylation that occur in ageing T cells are in transcriptionally repressed genes. However, the DNA methylation changes that are accompanied with gene expression changes affect many genes that are essential for the differentiation and function of T cells and shed light on the possible causes of the age-related decline in immune response. In addition, our study forms the basis to further evaluate the potential use of the identified DNA methylation changes as clinical markers of immunosenescence in older individuals.
Materials and Methods
Ethics statement
The study was approved by the Ethics Review Committee of Human Research of the University of Tartu, Estonia (permission no 206/T-4, date of issue 25.08.2011) and it was carried out in compliance with the Helsinki Declaration. All of the participants were older than 18 and a written informed consent to participate in the study was obtained from each individual prior to recruitment. All participants were healthy donors of the Estonian Genome Center of the University of Tartu. All methods were carried out in accordance with approved guidelines.
Purification of cell populations
Peripheral blood mononuclear cells (PBMC) were extracted using Ficoll-Paque (GE Healthcare) gradient centrifugation. CD8+ T-cells and CD4+ T-cells were extracted from PBMCs by consecutive positive separation using microbeads (CD4+ #130-045-101; CD8+ #130-045-201) and AutoMACS technology (Miltenyi Biotec) according to the manufacturer’s protocol. The purity of extracted CD4+ T-cells was 91–95% and for CD8+ T-cells 88–91%. All cell populations were collected and stored as cell pellets in a −80 °C freezer.
DNA extraction, bisulfite treatment and DNA methylation measurement
Genomic DNA was isolated from the purified cell pellets using the QIAmp DNA Mini Kit (Qiagen) according to the manufacturer’s protocol. DNA from whole blood was extracted by the salting-out method using 10 M ammonium acetate. The DNA was precipitated in isopropanol, washed in 70% ethanol, and finally resuspended in 1X TE buffer. The purity and concentrations of the DNA samples were measured by NanoDrop ND-1000 spectrophotometry. 500 ng of genomic DNA was treated with sodium bisulfite using the EZ DNA Methylation Kit (Zymo Research Corporation) according to the manufacturer’s instructions. DNA methylation analysis was performed using the Infinium Human Methylation 450 K BeadChip (Illumina). Quantitative analysis of DNA methylation was verified using Sequenom’s EpiTYPER T Complete Reagent Set and MassARRAY Analyzer 4 (Sequenom). DNA was amplified using bisulfite-converted DNA, Hot Start DNA Polymerase (Solis BioDyne) and specific primers, according to the EpiTYPER protocol. Primers were designed with Sequenom’s EpiDesigner program and are listed in Supplementary Table S4.
RNA extraction, labelling, hybridization, and qPCR
RNA was extracted from the purified T-cells using the miRNeasy Mini Kit combined with recommended RNase-free DNase I treatment (both from Qiagen). RNA from whole blood was purified using the MagMAX™ for Stabilized Blood Tubes RNA Isolation Kit. RNA was concentrated using the Heraeus vacuum centrifugation system without heating. RNA was labeled and amplified using the TargetAmp-Nano Labeling Kit for Illumina Expression BeadChip (Epicentre Biotechnologies) with SuperScript III Reverse Transcriptase (Life Technologies), followed by purification with the RNeasy MinElute Cleanup Kit (Qiagen). RNA quality was assessed after extraction and after labelling using an Agilent 2100 Bioanalyzer and Agilent RNA 6000 Nano Kit (all from Agilent Technologies). Labeled RNA was hybridized to the HumanHT-12 v4 Expression BeadChip (Illumina) according to the manufacturer’s instructions. Verification of the expression of changed genes in CD4+ and CD8+ T cells was performed by qPCR using primers listed in Supplementary Table S4.
Normalization of methylation data
Data pre-processing and quality control analyses were performed in R with the Bioconductor package minfi, using the original IDAT files extracted from the HiScanSQ scanner. ‘Raw’ pre-processing was used to convert the intensities from the red and the green channels into methylated and unmethylated signals. Beta values were computed using Illumina’s formula [beta = M/(M + U + 100)]. The difference in the distribution of beta values for type I and type II probes was corrected using “SWAN”58, a normalization method to address systematic changes between type I and type II probes. Detection p-values were obtained for every CpG probe in each sample. Failed positions were defined as signal levels lower than background from both the methylated and unmethylated channels.
Samples with detection p-value > 0.01 in more than 10% of the CpG sites and possibly contaminated or mixed up samples (determined using the 65 SNPs present on the methylation beadchip) were discarded. We mapped all the probe sequences to the human genome (build hg19) and discarded all probes that did not map uniquely, mapped to CpG sites with SNPs up to 3 bp upstream of the site, or mapped to the X/Y chromosomes. After data preprocessing, the total number of samples left for analysis was 296 (97+99+100). The total number of retained CpG sites was 399,653. The full data is stored at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE59065.
Differential methylation analysis
Before performing differential methylation analysis the effects of known confounding variables were removed. For samples from CD4+ and CD8+ cells the effect of sex and array was corrected for. In the case of PBLs the levels of erythrocytes, platelets, monocytes, lymphocytes and granulocytes that were measured by blood biochemistry were additionally included as covariates. The correction was performed by fitting a linear model with these covariates to every CpG site and using the residuals for further analysis. The differential methylation analysis of younger and older persons was performed using moderated t-test from the Bioconductor limma package59. CpG sites with an average methylation difference >0.05 between the younger and the older subjects, and false discovery rate (FDR) <0.05 were considered differentially methylated.
Differential expression analysis
Differentially expressed genes were identified using a moderated t-test from the Bioconductor limma package59. The same confounding factors used in the methylation analysis were applied for the different cell types. FDR <0.05 was used as threshold in all comparisons.
Gene Ontology analysis
All Gene Ontology enrichment analyses were performed using the g:Profiler web toolkit60 and R package GOsummaries (http://www.bioconductor.org/packages/release/bioc/html/GOsummaries.html). To display less redundant and more specific results, GOsummaries uses several non-default filtering options in g:Profiler. First, we limited the number of genes in a category; the upper limit of the number of genes was set to 1000 and the lower limit was 50 genes. Second, the program uses a “Best per parent” hierarchical filtering option to remove categories that are closely related on a GO tree. Finally, the results from “Cellular Component” and “Molecular Function” sections of GO were removed, as too general and not informative. The statistical significance threshold was 0.05 for the false discovery rate provided by g:Profiler.
Gene part overlaps
The CpG site associations with gene subregions were obtained from the annotation file provided by Illumina. In the file one site can be annotated to multiple regions if it is associated with more than one gene/island subregion.
Histone modification overlaps
Roadmap Epigenomics61 histone modification data of naïve and memory CD4+ and CD8+ T cells was downloaded in Wig format from http://www.roadmapepigenomics.org/data . The read counts were extracted from the files for all differentially methylated and 20,000 non-differentially methylated CpG sites using the rtracklayer R package62. To make the scores comparable between modifications and cell types, these were log2 transformed (using pseudocount 1) and converted into z-scores based on the background distribution of the 20,000 sites. The coefficients and confidence intervals shown in Fig. 3 were obtained from a linear model that predicted the scores using the direction of differential methylation. In order to account for the preferential distribution of histone modifications along the genes and CpG islands, this information was also included in the linear models.
Correlation between methylation and gene expression
The CpG sites were associated with genes based on annotated gene names (provided by Illumina). The gene associations to gene expression probes were obtained using gConvert tool of the g:Profiler web toolkit60. In the correlation plots differentially methylated/expressed genes were used (described above). The plots with gene transcripts were drawn using the Gviz package (http://www.bioconductor.org/packages/devel/bioc/html/Gviz.html), and were based on the known genes table from the UCSC genome browser, genome build hg19.
Additional Information
How to cite this article: Tserel, L. et al. Age-related profiling of DNA methylation in CD8+ T cells reveals changes in immune response and transcriptional regulator genes. Sci. Rep. 5, 13107; doi: 10.1038/srep13107 (2015).
Supplementary Material
Acknowledgments
This work was supported by Estonian Research Council grants IUT2-2 and IUT20-60, the University of Tartu for the Center of Translational Genomics (SP1GVARENG), the European Regional Development Fund in the framework of the Centre of Excellence in Genomics (EXCEGEN) and the Centre of Translational Medicine, and ERA-Net.Rus grant EGIDA.
Footnotes
Author Contributions L.T., L.M. and P.P. designed the experiments; L.T., K.K. and M.L. performed the cell sorting and prepared the samples for analysis; L.M. and A.M. designed and performed expression and methylation analysis; R.K., K.T., M.S. and S.K. designed and performed the computational analyses; L.T., M.L., R.K., K.T., L.M. and P.P. interpreted the data and wrote the manuscript. All authors provided input to the manuscript and all authors read and approved the final manuscript.
References
- Fulop T., Larbi A. & Pawelec G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front Immunol 4, 271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J. J., Li G., Yang Z. & Weyand C. M. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol 4, 131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S., Herndler-Brandstetter D., Weinberger B. & Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev 10, 362–369 (2011). [DOI] [PubMed] [Google Scholar]
- Pawelec G. et al. Immunosenescence and Cytomegalovirus: where do we stand after a decade? Immun Ageing 7, 13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder C. M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgheysari B. et al. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 81, 7759–7765 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer U. et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol 170, 2022–2029 (2003). [DOI] [PubMed] [Google Scholar]
- Franceschi C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908, 244–254 (2000). [DOI] [PubMed] [Google Scholar]
- Sansoni P. et al. The immune system in extreme longevity. Exp Gerontol 43, 61–65 (2008). [DOI] [PubMed] [Google Scholar]
- Zanni F. et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol 38, 981–987 (2003). [DOI] [PubMed] [Google Scholar]
- Wikby A., Månsson I. A., Johansson B., Strindhall J. & Nilsson S. E. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20-100 years of age. Biogerontology 9, 299–308 (2008). [DOI] [PubMed] [Google Scholar]
- Harries L. W. et al. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell 10, 868–878 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J. N., Gollapudi S., Sharman E. H., Jia Z. & Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell 9, 19–31 (2010). [DOI] [PubMed] [Google Scholar]
- Passtoors W. M. et al. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell 12, 24–31 (2013). [DOI] [PubMed] [Google Scholar]
- Marttila S. et al. Transcriptional Analysis Reveals Gender-Specific Changes in the Aging of the Human Immune System. Plos One 8, e66229 (2013). 10.1371/journal.pone.0066229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen T. et al. Cytomegalovirus (CMV)-dependent and -independent changes in the aging of the human immune system: A transcriptomic analysis. Experimental Gerontology 48, 305–312 (2013). [DOI] [PubMed] [Google Scholar]
- Garagnani P. et al. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell 11, 1132–1134 (2012). [DOI] [PubMed] [Google Scholar]
- Tserel L. et al. CpG sites associated with NRP1, NRXN2 and miR-29b-2 are hypomethylated in monocytes during ageing. Immunity & Ageing 11, 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. T. et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet 8, e1002629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C. et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet 5, e1000602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T. et al. An integrative multi-scale analysis of the dynamic DNA methylation landscape in aging. PLoS Genet 11, e1004996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklandt S. et al. Epigenetic predictor of age. PLoS One 6, e14821 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49, 359–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 14, R115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius L. E. et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 7, e41361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A. E. & Irizarry R. A. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol 15, R31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman E. A. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13, 86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokk K. et al. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol 15, R54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M. et al. DNA hypomethylation within specific transposable element families associates with tissue-specific enhancer landscape. Nat Genet 45, 836–841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich G. A. & Ilarregui J. M. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev 230, 144–159 (2009). [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Förster R., Lipp M. & Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- Taraban V. Y. et al. CD27 costimulation contributes substantially to the expansion of functional memory CD8(+) T cells after peptide immunization. Eur J Immunol 43, 3314–3323 (2013). [DOI] [PubMed] [Google Scholar]
- Hendriks J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 1, 433–440 (2000). [DOI] [PubMed] [Google Scholar]
- Hardie D. L. et al. The stromal cell antigen CD248 (endosialin) is expressed on naive CD8+ human T cells and regulates proliferation. Immunology 133, 288–295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. F. et al. Tissue-specific dysregulation of DNA methylation in aging. Aging Cell 9, 506–518 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan V. K. et al. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res 20, 434–439 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff A. E. et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res 20, 440–446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C. I. et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol 15, R24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. M. & Wagner W. Epigenetic-aging-signature to determine age in different tissues. Aging (Albany NY) 3, 1018–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson S. M., Riddell N. E. & Akbar A. N. Properties of end-stage human T cells defined by CD45RA re-expression. Current Opinion in Immunology 24, 476–481 (2012). [DOI] [PubMed] [Google Scholar]
- Koch S. et al. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing 5, 6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt P., Tee W. W. & Reinberg D. A double take on bivalent promoters. Genes Dev 27, 1318–1338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff C. J. Leveraging fluorinated glucosamine action to boost antitumor immunity. Curr Opin Immunol 25, 206–213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F. & Dimitroff C. J. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol 142, 107–116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovini R. et al. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol 179, 4283–4291 (2007). [DOI] [PubMed] [Google Scholar]
- Denton A. E., Russ B. E., Doherty P. C., Rao S. & Turner S. J. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci USA 108, 15306–15311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony D. A., Andrews D. M., Watt S. V., Trapani J. A. & Smyth M. J. Functional dissection of the granzyme family: cell death and inflammation. Immunol Rev 235, 73–92 (2010). [DOI] [PubMed] [Google Scholar]
- Scharer C. D., Barwick B. G., Youngblood B. A., Ahmed R. & Boss J. M. Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol 191, 3419–3429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zediak V. P., Johnnidis J. B., Wherry E. J. & Berger S. L. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol 186, 2705–2709 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burute M., Gottimukkala K. & Galande S. Chromatin organizer SATB1 is an important determinant of T-cell differentiation. Immunol Cell Biol 90, 852–859 (2012). [DOI] [PubMed] [Google Scholar]
- Krangel M. S. T cell development: better living through chromatin. Nat Immunol 8, 687–694 (2007). [DOI] [PubMed] [Google Scholar]
- Galande S., Purbey P. K., Notani D. & Kumar P. P. The third dimension of gene regulation: organization of dynamic chromatin loopscape by SATB1. Curr Opin Genet Dev 17, 408–414 (2007). [DOI] [PubMed] [Google Scholar]
- Notani D. et al. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol 8, e1000296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Leid M. & Rothenberg E. V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 329, 89–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylski G. K. et al. Disruption of the BCL11B gene through inv(14)(q11.2q32.31) results in the expression of BCL11B-TRDC fusion transcripts and is associated with the absence of wild-type BCL11B transcripts in T-ALL. Leukemia 19, 201–208 (2005). [DOI] [PubMed] [Google Scholar]
- Yui M. A. & Rothenberg E. V. Developmental gene networks: a triathlon on the course to T cell identity. Nat Rev Immunol 14, 529–545 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. M., Kaech S. M. & Staron M. M. The interface between transcriptional and epigenetic control of effector and memory CD8+ T-cell differentiation. Immunol Rev 261, 157–168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic J., Gordon L. & Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome Biol 13, R44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3 (2004). [DOI] [PubMed] [Google Scholar]
- Reimand J., Arak T. & Vilo J. g:Profiler–a web server for functional interpretation of gene lists (2011 update). Nucleic Acids Res 39, W307–W315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B. E. et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M., Gentleman R. & Carey V. Rtracklayer: an R package for interfacing with genome browsers. Bioinformatics 25, 1841–1842 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.