Abstract
AIM: To elucidate the potential biological role of miR-30b in gastric cancer and investigate the underlying molecular mechanisms of miR-30b to inhibit metastasis of gastric cancer cells.
METHODS: The expression of miR-30b was detected in gastric cancer cell lines and samples by reverse transcription-polymerase chain reaction. CCK-8 assays were conducted to explore the impact of miR-30b overexpression on the proliferation of gastric cancer cells. Flow cytometry was used to examine the effect of miR-30b on the apoptosis. Transwell test was used for the migration and invasion assays. Luciferase reporter assays and Western blot were employed to validate regulation of putative target of miR-30b.
RESULTS: The results showed that miR-30b was downregulated in gastric cancer tissues and cancer cell lines and functioned as a tumor suppressor. Overexpression of miR-30b promoted cell apoptosis, and suppressed proliferation, migration and invasion of the gastric cancer cell lines AGS and MGC803. Bioinformatic analysis identified the 3’-untranslated region of eukaryotic translation initiation factor 5A2 (EIF5A2) as a putative binding site of miR-30b. Luciferase reporter assays and Western blot analysis confirmed the EIF5A2 gene as a target of miR-30b. Moreover, expression levels of the EIF5A2 targets E-cadherin and Vimentin were altered following transfection of miR-30b mimics.
CONCLUSION: Our findings describe a link between miR-30b and EIF5A2, which plays an important role in mediating epithelial-mesenchymal transition.
Keywords: miR-30b, Gastric cancer, EIF5A2, Migration, Invasion
Core tip: In this study, we found that miR-30b expression was reduced in gastric cancer cell lines and in gastric cancer tissues. Moreover, we found that miR-30b inhibited gastric cancer cell proliferation, migration, invasion and promoted apoptosis by targeting EIF5A2. Restoration of miR-30b expression could enhance E-cadherin and β-catenin expression and suppress Vimentin expression by targeting EIF5A2 and eventually inhibit the epithelial-to-mesenchymal transition (EMT) process in gastric cancer cells, whereas knockdown of miR-30b promoted cell invasion and EMT in cancer cells.
INTRODUCTION
Metastasis to distant sites is the primary cause of death in patients with gastric cancer. Patients with advanced disease frequently develop recurrence and metastasis, even in early gastric cancer, the incidence of lymph node metastasis exceeds 10%[1]. However, the underlying molecular mechanism of metastasis is not entirely clear. Epithelial-to-mesenchymal transition (EMT) is a key molecular step during progression of gastric cancer to metastasis[2], and is associated with poor prognosis[3]. In this process, epithelial cancer cells in primary tumors lose cell-cell adhesion following E-cadherin repression and acquire a mesenchymal phenotype. This enhances the ability of cancer cells to metastasize and invade distant locations.
MicroRNAs (miRNAs) are small non-coding RNAs which negatively regulate gene expression. Various studies have described functional roles for miRNAs as oncogenes or tumor-suppressor genes. For example, miR-199a was found significantly upregulated in gastric cancer where it mediated an increase in cell proliferation and suppressed apoptosis[4]. miR-7 and miR-9 are important tumor suppressors which target various genes in gastric cancer[5,6]. Additionally, the miRNA expression profile in plasma from gastric cancer patients is different to that from normal individuals and may represent an early diagnostic biomarker for gastric cancer[7]. Furthermore, miR-15b and miR-16 could modulate the sensitivity of gastric cancer cells to chemotherapeutic drugs by regulating BCL2 expression[8]. All these suggest that miRNAs could serve as potential diagnostic biomarkers and therapeutic tools.
Accumulating evidence describes vital roles for many miRNAs in tumor initiation and metastasis. For example, miR-205 and the miR-200 family influence the EMT process during cancer metastasis[9]. Additionally, miR-7 can inhibit the EMT process in gastric cancer through targeting IGF1R expression[5]. In colorectal carcinoma, miR-30b directly targets the EMT-related gene SIX1 to impair metastasis of colorectal cancer cells[10]. Our current study adds to this knowledge by describing a role for miR-30b in the repression of gastric cancer cell metastasis.
The mechanisms underlying action of miR-30b on gastric cancer cell regulation have not yet been characterized. EIF5A2 functions as an oncogenic protein in many human cancers[11], and we have identified an miR-30b target site in the 3’-untranslated region (UTR) of EIF5A2 mRNA. Overexpression of miR-30b reduces levels of EIF5A2 mRNA and protein, affecting expression of downstream targets of EIF5A2. To the best of our knowledge, this is the first report of miR-30b directly targeting EIF5A2 to promote cellular apoptosis, and suppress proliferation, invasion, and metastasis of gastric cancer cells.
MATERIALS AND METHODS
Gastric cancer tissue specimens
Gastric cancer and corresponding non-tumorous gastric tissue specimens were collected from patients who underwent surgical resection at Peking Union Medical College Hospital (Beijing, China). No patients underwent chemotherapy or radiotherapy before surgery. A pathological diagnosis of gastric cancer was verified by at least two pathologists. All samples were frozen in liquid nitrogen and stored at -80 °C until use.
Cell culture and reagents
The human gastric cancer cell lines MKN45, MKN28, HGC27, and SGC7901, and human embryonic kidney (HEK) 293T cells were provided by the Cell Center of the Chinese Academy of Medical Sciences. The gastric cancer cell lines MGC803, N87, and AGS, and immortalized gastric mucosa GES-1 cells were from stores in our institute. HEK 293T cells were cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan Utah, United States) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, CA, United States). All other cell lines were grown routinely in RPMI-1640 medium with 10% FBS. All cells were cultured at 37 °C in a humidified incubator with 5% CO2.
SYBR green quantitative RT-PCR analysis
Total RNA from tissues and cell lines was extracted using Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. RNA was reverse-transcribed into cDNA with miRNA PrimeScript RT Enzyme (Takara, Dalian, China). Real-time RT-PCR was performed using SYBR Premix Ex Taq II (Takara), using U6 as the internal reference. PCR reactions were conducted using a 7300 Real-Time PCR system (ABI, United States) under the following conditions: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s, and 60 °C for 34 s. DNA primers specific for miR-30b and U6 small nuclear RNA were purchased from RiboBio (Guangzhou, China). The 2-ΔΔCt method was used to quantify relative miRNA expression. Experiments were performed in triplicate.
Transient transfection with miRNA mimic and inhibitor
Ectopic expression of miR-30b was performed by transfection with an miR-30b mimic or inhibitor (RiboBio) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. MiR-30b mimic control and inhibitor control were also synthesized by RiboBio. The sequences are as follows: miR-30b mimic, 5’-UGUAAACAUCCUACACUCAGCU-3’ (sense), and 3’-ACAUUUGUAGGAUGUGAGUCGA-5’ (antisense); miR-30b inhibitor, 5’-AGCUGAGUGUAGGAUGUUUACA-3’; miR-30b mimic control, 5’-UCACAACCUCCUAGAAAGAGUAGA-3’; miR-30b inhibitor control, 5’-UCACAACCUCCUAGAAAGAGUAGA-3’.
Cellular proliferation assays
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) (Dojindo) according to manufacturer’s instructions. Twenty-four hours after transient transfection of miRNA mimic or inhibitor, cells were harvested and seeded into 96-well plates at a density of 2 × 103 cells/well. Following incubation of cells for 24, 48, 72, or 96 h, the CCK-8 reagent (10 μL/well) was added to each well 1 h before the assay. The number of viable cells was assessed by measurement of OD450 values.
Apoptosis analysis
Quantification of apoptosis was conducted using an Annexin V-FITC Apoptosis Detection Kit (NeoBioscience, China). Cells were transfected with 50 nmol/L miR-30b mimic upon reaching 60% confluence in 6-well plates. Cells were then analyzed using a flow cytometer (BD Accuri C6).
Cell migration and invasion
Analyses of tumor cell migration and invasion were carried out using transwell chambers (8 μm Corning, United States). Forty-eight hours after transfection with miR-30b mimic or inhibitor, 2 × 105 AGS or MGC803 cells in serum-free medium were collected and seeded in an upper chamber containing a non-Matrigel coated membrane. Next, 500 μL medium with 20% FBS was added to the lower chamber. For the invasion assay, chambers were coated with extracellular Matrigel (BD Biosciences, United States). Cells were cultured at 37 °C in a humidified incubator with 5% CO2. Non-migrating or non-invading cells in the upper chamber were removed with a cotton swab and cells that migrated or invaded to the bottom chamber were fixed and stained with 0.1% crystal violet. Nine fields at × 100 magnification were randomly selected and cell numbers counted. The results were averaged among three independent experiments.
Plasmid construction and luciferase activity assay
The 751-bp fragment of wild-type (wt) EIF5A2-3’-UTR containing the putative miR-30b binding site was synthesized by PCR with the primers 5’-GCGCTCGAGTATTGTAGTCTGTTGGTGCC-3’ (forward) and 5’-AATGCGGCCGCTTTTCTTAAATCTTTGTTGC-3’ (reverse). This fragment was then inserted between the XhoI and NotI sites of the luciferase reporter vector pmiR-RB-REPORT™ (RiboBio). Mutations to the miR-30b seed sequence within the EIF5A2 3’-UTR were also generated. Constructs were validated by DNA sequencing. HEK293T cells were grown in 6-well plates and transiently co-transfected with 2 μg reporter plasmid and 50 nmol/L miRNA using Lipofectamine 2000. Twenty-four hours after transfection, luciferase activity was measured using the Dual-luciferase Reporter Assay System (Promega, United States). Firefly luciferase activity was normalized against the Renilla luciferase activity. Three independent experiments were performed in triplicate.
Western blot analysis
Cells were seeded in 6-well plates and transfected with miR-30b mimic or inhibitor for 72 h. Cells were then lysed in RIPA buffer (Genstar, Beijing, China) with 1% phenylmethylsulfonyl fluoride, and protein concentrations were determined by BCA assay. Samples were then denatured and 80 μg total proteins from each sample separated on a 10% SDS-PAGE gel and transferred onto PVDF membranes. Membranes were then blocked in 5% non-fat milk in Tris-buffered saline with 0.1% Tween-20 and incubated with primary antibody (rabbit anti-EIF5A2 monoclonal antibody, 1:1000; mouse anti-β-actin monoclonal antibody, 1:1000, Abcam, United States) overnight at 4 °C. The next day, membranes were washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. Signals were visualized using enhanced chemiluminescence reagent (Thermo) according to the manufacturer’s instructions.
Statistical analysis
To identify potential target genes of miR-30b, bioinformatics analysis was performed using an online miRNA target prediction database (Targetscan and miRNA.org).
Quantitative data were analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, United States). Experimental data are presented as mean ± SD. Differences between two groups were compared using a Student’s t-test and comparisons amongst three or more groups were made by analysis of variance. Differences were considered statistically significant when P < 0.05.
RESULTS
MiR-30b is downregulated in gastric cancer tissues
We used real-time PCR to examine miR-30b expression in human gastric adenocarcinoma and adjacent normal tissues. Expression of miR-30b was significantly decreased in gastric cancer tissue when compared with paired normal tissue in all 23 samples examined (P = 0.0016) (Figure 1A). Examining the relationship between clinicopathological factors and the expression of miR-30b showed that only lymph node metastasis was associated with low miR-30 expression (P = 0.021). No association was found between miR-30b expression and age, gender or Lauren type (Table 1). Furthermore, we identified reduced miR-30b expression in seven gastric cancer cell lines compared with that in human immortalized gastric mucosa cell line GES-1 (Figure 1B).
Figure 1.
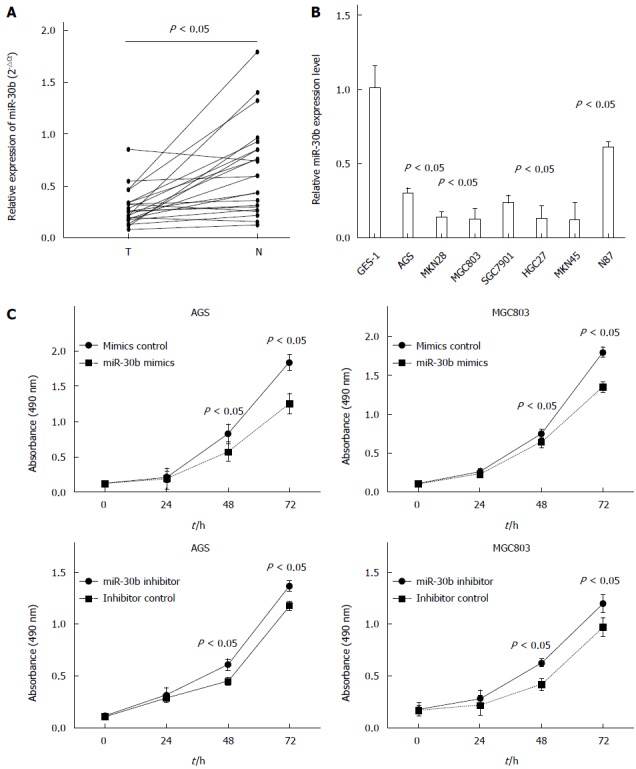
Expression levels of miR-30b in gastric tissue samples and gastric cell lines. A: MiR-30b expression was determined in 23 pairs of gastric cancer tissues compared with corresponding normal tissues by quantitative RT-PCR. Each sample was analyzed in triplicate and normalized to U6. T: tumor tissues; N: adjacent normal tissues; B: Lower miR-30b expression was observed in gastric cancer cell lines compared to that in GES-1; C: AGS and MGC803 cell proliferation was determined by the CCK-8 assay. Upregulation of miR-30b by transfection with mimic suppressed cell proliferation. Downregulation of miR-30b by transfection with inhibitor promoted cell proliferation. Data are displayed as mean ± SD.
Table 1.
Relationship between clinicopathological parameters and miR-30b expression
| Clinicopathologic parameter | Number of cases | 2-△△CT(mean) | P value |
| Age (yr) | 0.621 | ||
| ≥ 60 | 8 | 0.2078 ± 0.0285 | |
| < 60 | 15 | 0.2165 ± 0.0143 | |
| Gender | 0.427 | ||
| Male | 16 | 0.1951 ± 0.0198 | |
| Female | 7 | 0.2083 ± 0.0239 | |
| Lauren type | 0.371 | ||
| Intestinal type | 11 | 0.2148 ± 0.0316 | |
| Diffuse type | 12 | 0.1932 ± 0.0257 | |
| Lymph node metastasis | 0.021 | ||
| No | 9 | 0.2693 ± 0.0381 | |
| Yes | 14 | 0.1651 ± 0.0259 |
High expression of miR-30b suppresses gastric cancer cell proliferation
We next investigated the effects of miR-30b overexpression on cell growth using CCK-8 assay and synthetic miR-30b mimic or inhibitor that were transfected into AGS and MGC803 cell lines. Overexpression of miR-30b suppressed AGS and MGC803 cell growth, whereas miR-30b inhibitor enhanced gastric cancer cell proliferation (Figure 1C).
MiR-30b overexpression can induce gastric cancer cell apoptosis
We used flow cytometry to identify increased apoptosis in AGS and MGC803 cancer cells transfected with miR-30b mimic compared with control cells (P < 0.05, Figure 2). This suggests that apoptosis contributed to the growth-inhibitory properties of miR-30b.
Figure 2.
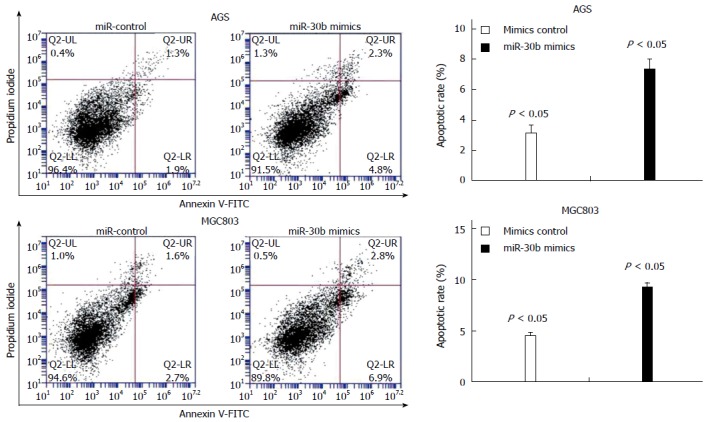
Effect of miR-30b on cell apoptosis. The histograms depict apoptosis of AGS cells and MGC803 cells transiently transfected with miR-30 mimic or control.
Re-expression of miR-30b suppresses gastric cancer cell migration and invasion
AGS and MGC803 cells transfected with 50 nmol/L miR-30b underwent reduced migration and invasion compared with control cells. Conversely, we detected increased migration and invasion in AGS and MGC803 cells transfected with an antisense oligonucleotide inhibitor of miR-30b (Figure 3). These results indicate that miR-30b attenuated gastric cancer cell migration and invasion in vitro.
Figure 3.
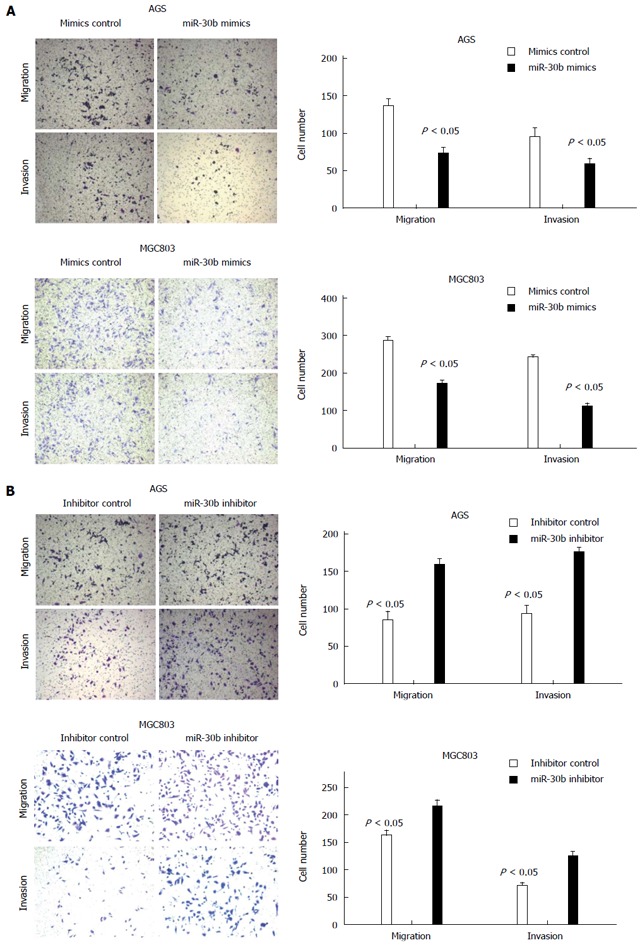
Effect of miR-30b on the migration and invasion of AGS and MGC803 cells in a transwell assay. A: Overexpression of miR-30b notably inhibited the migration and invasion of AGS and MGC803 cells; B: The migration and invasion abilities of AGS and MGC803 cells were dramatically increased after miR-30b inhibitor treatment. The bar shows the average ± SD of three independent experiments.
EIF5A2 is a candidate target gene of miR-30b
We conducted a bioinformatics analysis to identify potential targets of miR-30b using the online tools miRanda and TargetScan. EIF5A2 mRNA was found to contain a 3’-UTR element complementary to miR-30b, and the binding site of miR-30b in the 3’-UTR of EIF5A2 is highly conserved across species (Figure 4A and B). Therefore, we cloned the region of the EIF5A2 3’-UTR containing this complementary site into a luciferase reporter vector. Luciferase activity levels in HEK293T cells transfected with this construct and miR-30b mimic were significantly decreased compared with control. However, luciferase activity in cells transfected with reporter constructs harboring mutations at the suspected miR-30b target site was unaffected by co-transfection with miR-30b (Figure 4C). These results indicate that the 3’-UTR of EIF5A2 was targeted by miR-30b.
Figure 4.
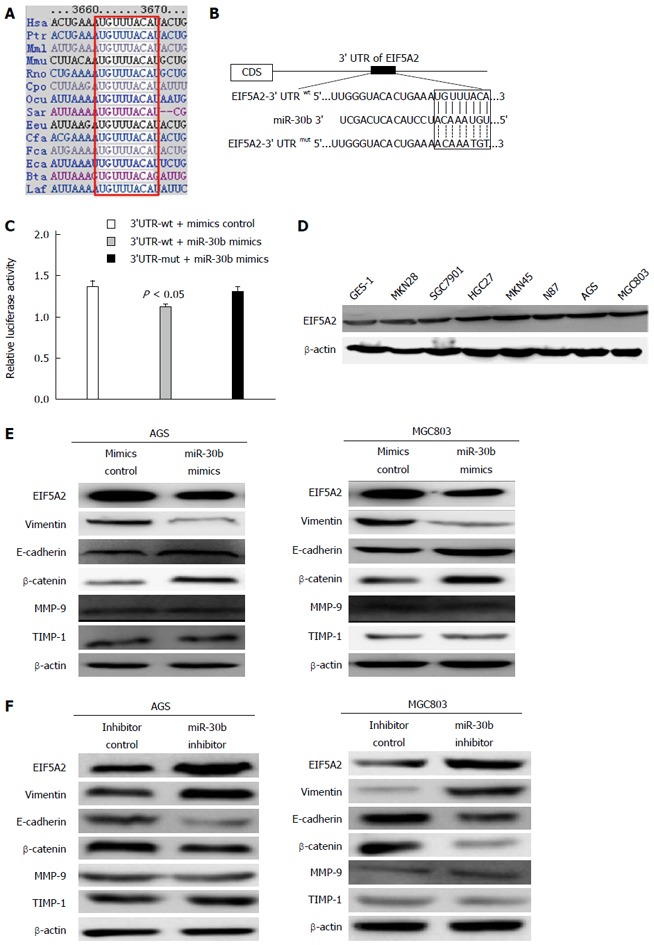
miR-30b decreases eukaryotic translation initiation factor 5A2 expression by targeting its 3’-UTR. A: The binding site of miR-30b in the 3’-UTR of eukaryotic translation initiation factor 5A2 (EIF5A2) is highly conserved across species; B: The putative binding sites for miR-30b was found in the 3’-UTR of EIF5A2 at 3664-3671bp; C: miR-30b mimic downregulated luciferase activities controlled by wild-type 3’-UTR of EIF5A2, but did not affect luciferase activity controlled by mutant 3’-UTR of EIF5A2; D: The expression levels of EIF5A2 protein in different gastric cell lines; E: The expression levels of EIF5A2 and of its downstream genes were detected by Western blot analysis in AGS and MGC803 cells transfected with the miR-30b mimic or control for 48 h. β-actin was used as an internal control; F: Western blot analysis of EIF5A2 and its downstream genes transfection of miR-30b inhibitor in AGS and MGC803 cells.
Downregulation of EIF5A2 by miR-30b promotes EMT
Western blot analysis identified significantly higher expression of EIF5A2 in all seven gastric cancer cell lines examined compared with GES-1 cells (Figure 4D).
We next examined the influence of miR-30b by measuring EIF5A2 protein levels following transfection of AGS and MGC803 cells with miR-30b mimic or inhibitor. Cells transfected with miR-30b mimic had significantly lower EIF5A2 protein levels compared with control (Figure 4E). Furthermore, transfection of miR-30b mimic led to increased expression of the epithelial marker E-cadherin and β-catenin and reduced expression of the mesenchymal marker Vimentin, whereas silencing miR-30b suppressed E-cadherin and β-catenin expression, and induced vimentin expression in cancer cells (Figure 4F). In addition, transfection with miR-30b mimic or inhibitor had no effect on the MMP-9 and TIMP-1, indicating that miR-30b suppressed cancer cell metastasis via downregulation of EIF5A2. These results suggest that miR-30b enhances E-cadherin and β-catenin expression by targeting EIF5A2 and eventually inhibits the EMT process in gastric cancer cells.
DISCUSSION
Increased expression of miR-30b has been identified in multiple malignancies including parathyroid carcinoma[12], medulloblastoma[13], and oral squamous cell cancer[14]. These findings support a role for miR-30b as an oncogene in these tumors. However, miR-30b may also function as a tumor suppressor. Reduced expression of miR-30b has been found in various human cancers, including colorectal cancer[10,15], non-small cell lung cancer[16], and prostate cancer[17]. These studies found that miR-30b could inhibit cancer cell proliferation and/or suppress cancer cell invasion and migration. Additionally, Ueda et al[18] found that miR-30b was significantly downregulated in 184 gastric cancers compared with 169 non-tumor mucosa samples. Moreover, restoration of miR-30b expression can inhibit gastric cancer cell migration and increase gastric cancer cell apoptosis[19,20]. This decreased expression of miR-30b in gastric cancer may result from miR-30b promoter methylation[20]. Taken together, these findings indicate that miR-30b can act as either an oncogene or a tumor suppressor depending on the circumstance.
We have identified low expression of miR-30b in gastric cancer specimens compared with adjacent non-cancerous tissues using real-time PCR-based miRNA assays. At the cellular level, miR-30b overexpression inhibited cancer cell proliferation, promoted cellular apoptosis, and decreased cancer cell migration and invasion. Additionally, gastric cancer cells transfected with miR-30b inhibitor exhibited increased growth, migration, and invasion compared with controls. Taken together, these data suggest that miR-30b plays a tumor suppressor role in gastric cancer.
A multiple-to-multiple relationship exists between miRNAs and their targets in gastric cancer, suggesting that their regulation is complex[21]. Members of the miR-30 family exert various effects in tumors from different tissues. In multiple myeloma, miR-30 family members are downregulated, which results in enhanced expression of BCL9 and subsequent promotion of tumor cell proliferation and migration[22]. Recently, two studies investigating the role of miR-30b in colorectal carcinoma development have identified that the oncogenes KRAS, PIK3CD, BCL9, and the EMT-related gene SIX1 are all targets of miR-30b[10,15]. Moreover, miR-30b expression in clinical samples was inversely correlated with the above genes. Additionally, Zhong et al[16] have reported that miR-30b is involved in non-small cell lung cancer carcinogenesis through downregulating the Ras superfamily member Rab18. Last, miR-30b inhibits expression of plasminogen activator inhibitor (PAI-1) in gastric cancer, thereby suppressing tumor growth[19]. So, more targets of miR-30b should be validated to investigate its function in gastric cancer.
The loss of miR-30b expression influences gastric cancer metastasis through altered regulation of miR-30b target gene expression. We employed a bioinformatics approach and luciferase reporter assay to identify EIF5A2 as a critical novel target of miR-30b. Western blot analysis revealed that miR-30b overexpression resulted in upregulation of E-cadherin and β-catenin and downregulation of Vimentin, demonstrating that miR-30b could suppress EMT in gastric cancer. However, no change was observed of MMP-9 and TIMP-1 levels after transfection with miR-30b mimic or inhibitor in cancer cells. These results indicated that miR-30b inhibits gastric cancer cell invasion and migration not through the MMP-9 or TIMP-1.
EFI5A2 is a potentially important tumor promoting molecule. Several studies have described an oncogenic role for EIF5A2 in multiple tumor types, including esophageal squamous cell carcinoma[23], hepatocellular carcinoma[24], bladder carcinoma[25], ovarian carcinoma[26], and colorectal carcinoma[27]. Additionally, EIF5A2 overexpression can initiate tumor formation, promote cancer cell growth, and contribute to cancer cell metastasis. Furthermore, high levels of EIF5A2 indicate a more advanced clinical stage in ovarian[26] and hepatocellular carcinomas[28]. Tang et al[24] found that EIF5A2 could induce EMT in hepatocellular carcinoma by activating RhoA/Rac1 and downregulating epithelial markers including E-cadherin and β-catenin. Overexpression of EIF5A2 can also promote EMT by regulating MTA1 through c-myc in human colorectal carcinoma[29]. In our previous study, we found that EIF5A2 was overexpressed in gastric cancer compared with matched adjacent non-tumor mucosal tissues[30]. Meanwhile, knockdown of EIF5A2 can suppress MKN28 and HGC27 cell proliferation, migration, and invasion by inhibiting EMT, and E-cadherin levels were upregulated and vimentin levels were downregulated after transfection with EIF5A2 siRNA[30,31]. Together, these findings support an oncogenic role for EIF5A2.
To the best of our knowledge, this is the first report that miR-30b directly regulates EIF5A2. miR-30b has a functional role in suppressing gastric cancer metastasis by impairing cellular migration and invasion. Downregulation of EIF5A2 by miR-30b could increase E-cadherin and β-catenin levels and reduce Vimentin expression. However, it should be noted that this study is based on a small number of samples and no experiments in relevant animal models were conducted. Therefore, future studies are necessary to elaborate upon our current findings. Overall, this report sheds new light on the role of miR-30b in gastric cancer development, and supports the targeting of miR-30b as a potentially effective therapeutic strategy for gastric cancer in the future.
COMMENTS
Background
Gastric cancer is one of the leading causes of cancer-related death worldwide. MicroRNAs (miRNAs) play an important role in gastric cancer carcinogenesis and tumor progression by negatively regulating oncogenes and tumor suppressors. However, the precise biological role of miRNAs in mediating metastasis remains relatively unexplored.
Research frontiers
MicroRNAs (miRNAs) are small non-coding RNAs which negatively regulate gene expression. Various studies have described functional roles for miRNAs as oncogenes or tumor-suppressor genes. Accumulating evidence describes vital roles for many miRNAs in tumor initiation and metastasis. For example, miR-7 can inhibit the epithelial-mesenchymal transition (EMT) process in gastric cancer through targeting IGF1R expression. In colorectal carcinoma, miR-30b directly targets the EMT-related gene SIX1 to impair metastasis of colorectal cancer cells. However, the role of miR-30b in gastric cancer progression and metastasis is still largely unknown and the molecular mechanism needs further exploration.
Innovations and breakthroughs
The authors found that miR-30b is downregulated in gastric cancer tissues and cancer cell lines and functions as a tumor suppressor. Overexpression of miR-30b can promote cell apoptosis, and suppress proliferation, migration, and invasion of the gastric cancer cell lines AGS and MGC803. Luciferase reporter assays and Western blot analysis confirmed the EIF5A2 gene as a target of miR-30b. Moreover, miR-30b enhances E-cadherin and β-catenin expression by targeting EIF5A2 and eventually inhibits the EMT process in gastric cancer cells, thus providing a valuable target for cancer therapy.
Applications
In this study, the authors found that miR-30b is significantly downregulated in gastric cancer tissues and cell lines. Increased miR-30b expression reduced cancer cell migration and invasion, and promoted cell apoptosis. They also found that suppression of EIF5A2 by miR-30b increased E-cadherin expression and partially reversed the EMT. All these provide insight into the specific role of miR-30b in EMT and tumor metastasis. The authors propose that miR-30b may be a novel target for the treatment of gastric cancer.
Terminology
MicroRNAs (miRNAs) are a novel class of small, non-coding endogenous RNAs that regulate gene expression by directing their target mRNAs for degradation or translational repression. EMT is a biological process during tumor development by which epithelial cells acquire mesenchymal, fibroblast-like properties and show reduced intercellular adhesion and increased motility.
Peer-review
The authors have described a putative role of miR-30b in regulating EIFA2 expression and function. The article is well organized and well written. The methods used are well studied and appropriate for the experimental design.
Footnotes
Supported by Beijing Municipal Natural Science Foundation of China, No. 7132209; and The Capital Health Research and Development of Special, No. 2014-3-4014.
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 12, 2015
First decision: March 26, 2015
Article in press: July 8, 2015
P- Reviewer: Klempner SJ, Li Y, Zhang J S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel) 2011;3:2141–2159. doi: 10.3390/cancers3022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Katoh M. Epithelial-mesenchymal transition in gastric cancer (Review) Int J Oncol. 2005;27:1677–1683. [PubMed] [Google Scholar]
- 4.Wang Z, Ma X, Cai Q, Wang X, Yu B, Cai Q, liu B, Zhu Z, Li C. MiR-199a-3p promotes gastric cancer progression by targeting ZHX1. FEBS Lett. 2014;588:4504–4512. doi: 10.1016/j.febslet.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–1372. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 6.Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo T, Yuasa Y. MiR-9 downregulates CDX2 expression in gastric cancer cells. Int J Cancer. 2011;129:2611–2620. doi: 10.1002/ijc.25923. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 9.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Xu Z, Qin H, Gao Z, Gao L. miR-30b regulates migration and invasion of human colorectal cancer via SIX1. Biochem J. 2014;460:117–125. doi: 10.1042/BJ20131535. [DOI] [PubMed] [Google Scholar]
- 11.Wang FW, Guan XY, Xie D. Roles of eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci. 2013;9:1013–1020. doi: 10.7150/ijbs.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbari R, Holloway AK, He M, Khanafshar E, Clark OH, Kebebew E. Identification of differentially expressed microRNA in parathyroid tumors. Ann Surg Oncol. 2011;18:1158–1165. doi: 10.1245/s10434-010-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Ryan SL, Elliott DJ, Bignell GR, Futreal PA, Ellison DW, Bailey S, Clifford SC. Amplification and overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at 8q24.22-q24.23 in medulloblastoma. PLoS One. 2009;4:e6159. doi: 10.1371/journal.pone.0006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao C, Yu Y, Yu L, Pei Y, Feng Q, Chu F, Fang Z, Zhou Y. Amplification and up-regulation of microRNA-30b in oral squamous cell cancers. Arch Oral Biol. 2012;57:1012–1017. doi: 10.1016/j.archoralbio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY, Cui YM, Qi L, Wu P, Jiao HL, Xie YJ, et al. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol. 2014;232:415–427. doi: 10.1002/path.4309. [DOI] [PubMed] [Google Scholar]
- 16.Zhong K, Chen K, Han L, Li B. MicroRNA-30b/c inhibits non-small cell lung cancer cell proliferation by targeting Rab18. BMC Cancer. 2014;14:703. doi: 10.1186/1471-2407-14-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao CJ, Martiniez A, Shi XB, Yang J, Evans CP, Dobi A, deVere White RW, Kung HJ. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2014;33:2495–2503. doi: 10.1038/onc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao XH, Guo G, Zou QM, Xiao B. miR-30b, down-regulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS One. 2014;9:e106049. doi: 10.1371/journal.pone.0106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao F, Zhang K, Gong P, Wang L, Hu J, Lu S, Fan H. Decreased miR-30b-5p expression by DNMT1 methylation regulation involved in gastric cancer metastasis. Mol Biol Rep. 2014;41:5693–5700. doi: 10.1007/s11033-014-3439-4. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013;8:e62589. doi: 10.1371/journal.pone.0062589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao JJ, Lin J, Zhu D, Wang X, Brooks D, Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al. miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res. 2014;74:1801–1813. doi: 10.1158/0008-5472.CAN-13-3311-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, et al. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701–1713.e9. doi: 10.1053/j.gastro.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- 25.Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM, Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, et al. EIF5A2 predicts outcome in localised invasive bladder cancer and promotes bladder cancer cell aggressiveness in vitro and in vivo. Br J Cancer. 2014;110:1767–1777. doi: 10.1038/bjc.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua WF, Wu HM, Kung HF, Zeng YX, Guan XY. Expression and amplification of eIF-5A2 in human epithelial ovarian tumors and overexpression of EIF-5A2 is a new independent predictor of outcome in patients with ovarian carcinoma. Gynecol Oncol. 2009;112:314–318. doi: 10.1016/j.ygyno.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, Kung HF, Guan XY. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39:80–86. doi: 10.1016/j.humpath.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, Dong S, Guan XY, Poon RT, Luk JM. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127:968–976. doi: 10.1002/ijc.25100. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymaltransition. Gut. 2012;61:562–575. doi: 10.1136/gutjnl-2011-300207. [DOI] [PubMed] [Google Scholar]
- 30.Meng QB, Kang WM, Yu JC, Liu YQ, Ma ZQ, Zhou L, Cui QC, Zhou WX. Overexpression of eukaryotic translation initiation factor 5A2 (EIF5A2) correlates with cell aggressiveness and poor survival in gastric cancer. PLoS One. 2015;10:e0119229. doi: 10.1371/journal.pone.0119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng QB, Yu JC, Kang WM, Ma ZQ, Zhou L, Ye X, Cao ZJ, Tian SB. [Effects of eukaryotic translation initiation factor 5A2 down-regulation by small interfering RNA on aggressiveness of MKN28 human] Zhongguo Yixue Kexueyuan Xuebao. 2014;36:482–487. doi: 10.3881/j.issn.1000-503X.2014.05.005. [DOI] [PubMed] [Google Scholar]


