FIGURE 3.
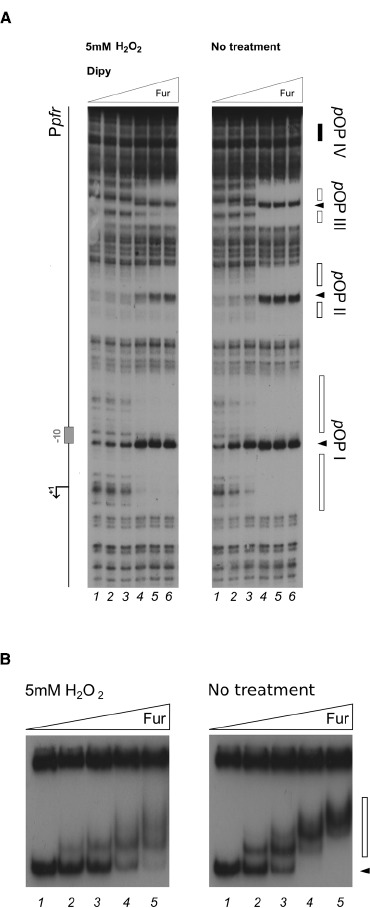
Differential Fur binding on the apo-repressed pfr promoter in response to hydrogen peroxide. (A) Considering the extreme affinity of the protein for the Ppfr OPI element, we performed a footprinting at lower Fur concentrations. The protein was preincubated with the Ppfr probe in binding buffer containing 1 mM DTT and 150 μM Dipyridyl for 10 min, then 5 mM H2O2 was added and the binding reaction was incubated for further 10 min (left panel); the control reaction (right panel) was treated with the same volume of water. Legends and symbols as in Figure 2. Lanes 1–5: 0, 0.3, 0.6, 3.3, and 8 nM Fur dimer, respectively. (B) EMSA performed on the pfr promoter probe with 1 mM DTT or 5 mM H2O2, in the presence of 150 μM Dipyridyl. Lanes 1–5: 0, 0.83, 1.7, 3.4, and 6.8 nM Fur dimer. A black arrowhead indicates the free probe, the white bar denotes the ladder generated by subsequent Fur binding events on the probe.
