Organization of G Protein Cascades: Lessons From Photoreceptors
In order to survive and function properly, mammalian cells send and receive a vast number of signals that are used to adjust their behavior in response to changes in the environment. Among the multiple receptor systems that cells utilize for this purpose, the most prominent role is undoubtedly played by G protein-coupled receptors (GPCRs). These cell surface receptors respond to an incredibly wide repertoire of ligands, including ions, peptides, lipids, neurotransmitters and light. G protein-coupled receptors constitute the largest family in mammalian genomes, accounting for approximately 3% to 4% of all genes.1 Despite their versatility, the organization of all known GPCRs is rather conserved; they share a common seven transmembrane topology and the ability to activate intracellular heterotrimeric G proteins.2 Ligand binding causes GPCRs to undergo a conformational change, which is sensed intracellularly by G proteins, causing them to release guanosine diphosphate (GDP) in exchange for guanosine triphosphate (GTP). Nucleotide binding occurs on the Gα subunit and results in its dissociation from the Gβγ subunits. In their dissociated state, both Gα-GTP and free Gβγ are able to interact with and regulate the activity of downstream effectors, including proteins key to cellular homeostasis, such as ion channels, kinases, and second messenger-producing/degrading enzymes. This signaling is terminated upon the hydrolysis of GTP by the Gα subunit, causing its inactive Gα-GDP form to reassociate with the Gβγ subunit.
Much of what we know about the functional organization of GPCR systems is derived from the phototransduction cascade of vertebrate photoreceptors, one of the first and the best-studied G protein pathways. As a result, the lessons learned in the study of photoreceptors have had a tremendous impact on our understanding of GPCR biology and will likely continue to guide research on G protein cascades for years to come. The main sequence of the events in phototransduction is now well established and has been the subject of several excellent reviews.3–6 In prototypic rod photoreceptors, light causes a conformational change in the photosensitive GPCR rhodopsin by inducing isomerization of the receptor-bound inverse agonist 11-cis retinal into the full agonist all-trans retinal. Photoexcited rhodopsin activates G protein transducin, which in turn dissociates into Gαt1-GTP and Gβ1γ1 subunits. Activated Gαt1-GTP binds to its effector enzyme—the gamma subunit of phosphodiesterase, type 6 (PDE6γ)—and relieves the inhibitory constraint that this subunit has on the catalytic PDE6αβ subunits, which leads to the hydrolysis of the second messenger cGMP. The declining concentrations of cGMP allow the opening of cGMP-gated ion (CNG) channels on the plasma membrane, leading to cellular hyperpolarization and the resulting inhibition of neurotransmitter release. All components of the phototransduction cascade are delegated to a special compartment of the cell called the outer segment, which is essentially an elaboration of the primary cilia. Thus, the phototransduction cascade is highly compartmentalized, revealing the first lesson from this GPCR cascade. The second lesson is provided by studies on the mechanisms that allow photoreceptors to quickly recover from excitation, a property that is essential for achieving the high temporal resolution of our vision. This process requires the deactivation of phototransduction, which involves the termination of both rhodopsin and transducin signaling.7,8 One of the major breakthroughs in the field was the demonstration that transducin deactivation is the rate-limiting step in the termination of phototransduction reactions.9 Transducin, as well as all other G proteins, has a very slow GTP hydrolysis rate, with kinetics that are insufficient to explain the physiologically relevant speed of photoresponse termination. The timely deactivation of transducin requires the contribution of another element of the GPCR cascade, type 9 regulator of G protein signaling (RGS9), which functions to speed up the rate of GTP hydrolysis of this G protein.10,11 Type 9 regulator of G protein signaling belongs to a family of RGS proteins that consists of more than 30 members ubiquitously expressed in all cells and involved in the regulation of GPCR signaling.12 Thus, the second lesson learned from the organization of the phototransduction cascade is the key involvement of RGS proteins for achieving physiologically relevant timing.
In photoreceptors, RGS9 does not act alone but requires the contribution of two proteins with which it forms a tight complex, and which are now considered to be its bona fide subunits. The first protein, an atypical member of the G protein family, type 5 beta subunit (Gβ5), is required for ensuring the correct folding and stability of the complex,13,14 with additional contributions in guiding RGS9 to selectively recognize its correct substrate, the Gαt1-PDE6γ complex, instead of free Gαt1.15,16 The second molecule, a SNARE-like transmembrane protein named RGS9 anchor protein (R9AP), delivers the complex to the outer segments of the photoreceptors, positioning it on the disc membranes17,18; R9AP also plays an essential role in determining the proteolytic stability of the complex.19 Work on the organization and functional regulation of the RGS complex in photoreceptors by Vadim Arshavsky and Theodore Wensel received the Proctor award in 201320 and highlights the third key lesson: The components are scaffolded together in tight macromolecular complexes, with intimate interplay between components that ultimately defines their function in GPCR regulation. With such deep mechanistic understanding of the vertebrate phototransduction cascade, one of the key challenges is to test how general these principles are and whether they can be used as a universal template for understanding the organization and functional regulation of other much less well-studied GPCR cascades.
The G Protein Cascade at the First Visual Synapse
The initial response to light generated by photoreceptors would not be of much use unless it can be transmitted across the neural circuit in the retina and eventually to the visual cortex in the brain. To do so, photoreceptors form synaptic connections with downstream neurons, bipolar cells (BCs). Excitation of photoreceptors by light inhibits the release of the neurotransmitter glutamate by their axonal terminals, and these changes in the glutamate concentration in the synaptic cleft are differentially sensed by two classes of downstream BCs. OFF-bipolar cells signal in the dark and are inactivated upon light stimulation, whereas the ON type of BCs carry excitatory signaling to the responses produced by photoreceptors in the light.21,22 Although cone photoreceptors responding to high-light intensities signal via both of these pathways, rods that are active under low illumination relay information exclusively to ON-BCs, making these cells essential for vision in dim light.
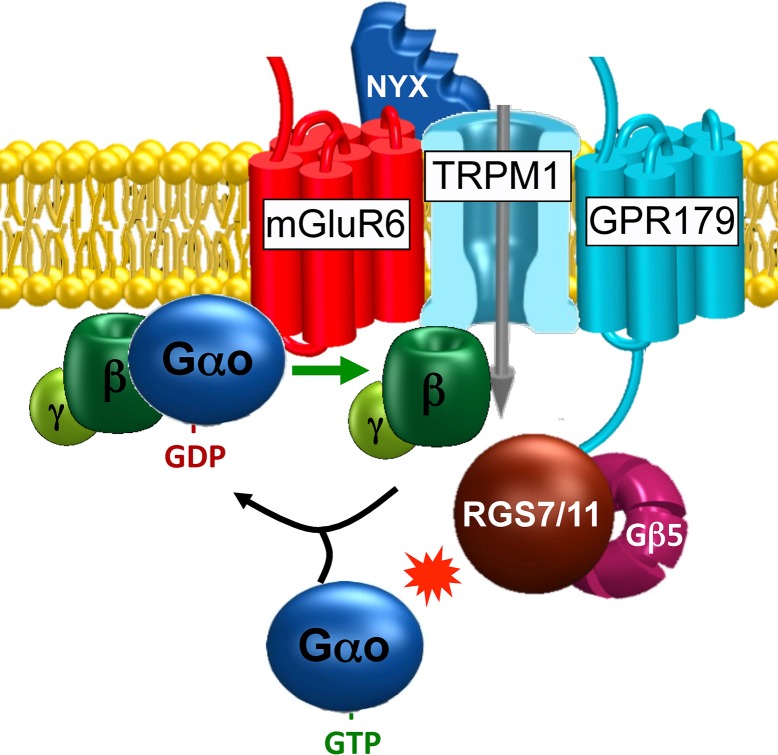
Remarkably, ON-BC neurons utilize a GPCR pathway that is reminiscent of the phototransduction for synaptic communication with the photoreceptors, pairing two G protein signaling systems in these synaptically connected cells. It has been established that the glutamate receptor of these cells is mGluR623,24 and that it is coupled via the G protein Go25,26 to the opening of the cation channel recently identified as TRPM1.27–29 In the dark, the pathway is constantly activated, holding the channels closed and causing the cell to hyperpolarize (Fig. 1). A decrease in glutamate release by photoreceptors during the light response reduces mGluR6 activation, triggering Go deactivation and the opening of TRPM1 channels and thus causing excitatory depolarization.30,31 Because synaptic transmission to ON-BCs is the primary pathway for rods to communicate their signal to the retinal circuitry, the inability to transfer the signal at this point results in the loss of scotopic vision, the condition in humans known as congenital stationary night blindness (CSNB).32 In the clinic, CSNB diagnosis is normally confirmed by performing ERG, an electrophysiological recording of light-induced changes in the field potential as an electrical signal propagates through retinal neurons. In normal subjects, a typical two-component waveform is observed: an a-wave that reflects the hyperpolarizing activity of photoreceptors and a b-wave that reflects ON-BC depolarization. In patients with CSNB, the b-wave is eliminated, indicating deficits in synaptic transmission.33 The mapping of mutations to genes in patients with this disease manifesting in either complete or incomplete elimination of the b-wave has proven to be highly insightful for identifying the molecular players essential for this synaptic communication. In addition to known elements, such as mGluR634,35 and TRPM1,36–38 as well as presynaptic calcium channels that regulate glutamate release,39,40 these studies have revealed several new proteins, including nyctalopin,41 orphan receptor GPR179,42,43 and cell adhesion-like protein LRIT3,44 the function of which in the ON-BC G protein signaling cascade is only beginning to be understood. A complementary and highly productive effort in the field has been provided by the phenotyping of a collection of mouse strains with random or targeted mutations for the absence of the ERG b-wave. This has led to the identification of many of the same genes, including nyctalopin,45 TRPM1,27–29 GPR179,42 LRIT3,46 and mGluR6,47 but also several unique players, including Gαo,25 Gβ3,48 and Gβ5,49 with essential roles in the synaptic transmission to ON-BCs.
Figure 1.
Organization of the G protein signaling cascade at the dendritic tips of retina ON-bipolar neurons.
Elucidating the Role of RGS Proteins in ON-BC Synaptic Transmission
Providing that the lessons gleaned from studies on the phototransduction cascade can be used as a guide, the G protein cascade in ON-BC neurons misses a critical element: an RGS protein. Indeed, work by Sampath and Rieke50 demonstrated that GTP hydrolysis is required for the onset of the ON-BC response; and using reconstituted system, Noga Vardi's51 group showed that mGluR6 signaling to ion channels can, in principle, be modulated by RGS proteins. Furthermore, the discovery that the elimination of Gβ5 produces a characteristic lack of the b-wave49 has brought attention to the R7 family of RGS proteins as possible regulators of ON-BC signaling. Similar to the case in photoreceptors, Gβ5 forms complexes with RGS proteins by irreversibly binding to a G-gamma-like domain in 3 other RGS9-like proteins, RGS6, RGS7, and RGS11, which form the R7 RGS family.52 As with RGS9 in photoreceptors, the formation of this complex is essential for the proteolytic stability of other R7 RGS members in the rest of the nervous system, as the knockout of Gβ5 in mice drastically reduces their expression.14 The interest in this RGS family has been further fueled by the demonstrated role of RGS9 in phototransduction, and it seems logical to delegate a related RGS protein to control the G protein cascade in the synaptically connected neuron. Our group has profiled the expression of R7 family members in the retina and found RGS7 and RGS11 to be specifically enriched in the synaptic layer, the site where photoreceptors make contacts with bipolar cells.53 This finding was independently corroborated by several investigators including the groups of Catherine Morgans,54 Theodore Wensel,55 and Jason Chen.56 To test the possible involvement of RGS7 and RGS11 in ON-BC signaling, mouse models with a disruption in the respective genes have been used. Elimination of RGS11 or RGS7 alone did not have a substantial effect on the b-wave,56–59 causing only minor alterations in the time required to mount a maximal response, which is inconsistent with their major role in the regulation of mGluR6 signaling, which would be predicted to produce major effects akin to what is observed in Gβ5 knockouts. Furthermore, combining RGS11 elimination with hypomorphic mutation in RGS7 did not largely exacerbate the phenotype, leading to the conclusion that neither RGS7 not RGS11 alone sufficiently impacts the mGluR6 signaling cascade of ON-BC cells. The breakthrough came when a true null allele of RGS7 was combined with the RGS11 knockout.60,61 Mice lacking both of these RGS proteins exhibited a complete lack of the ERG b-wave and the responsiveness of ON-bipolar cells to flashes of light. However, the ON-bipolar neurons exhibited a residual response when bright steps of light were applied to the retina for a prolonged period of time.61 The onset of the depolarizing response in retinas lacking RGS7 and RGS11 proteins was much slower than in wild type, suggesting that these proteins control the timing and sensitivity of TRPM1 channel activation. On the basis of these findings, we proposed a model in which RGS proteins in ON-bipolar neurons oppose the stimulatory action of mGluR6 to set the balance of G protein activation, such that in the dark, activated Go is produced in slight excess to effectively open TRPM1 channels. Light changes this balance, decreasing mGluR6 activity and allowing RGS proteins to deactivate Go below a certain threshold, such that TRPM1 channels begin to deactivate, generating a depolarizing response. When RGS proteins are completely eliminated, the balance is shifted to further favor Go activation, increasing the pressure to keep TRPM1 channels closed. The transient reduction in mGluR6 activity induced by light does not allow for a decrease in active Go to the action threshold that is rapid enough to open an appreciable number of channels. Hence, the opening probability of TRPM1 channels is decreased below the level needed for producing a depolarizing response. This makes the RGS proteins RGS7 and RGS11 key factors in the regulation of the mGluR6 cascade in ON-bipolar neurons, ultimately contributing to synaptic transmission. In summary, the lessons learned from photoreceptors regarding RGS involvement appear to also apply to the GPCR cascade in ON-bipolar neurons.
Components of the ON-BC Signaling Cascade Form Macromolecular Complexes
Recent studies on the organization of the mGluR6 signaling cascade of ON-BCs are increasingly mirroring another important lesson from phototransduction, which pertains to the intimate integration of key signal transduction components. Following identification of TRPM1 as an effector channel in the pathway, studies have focused on investigating its protein-protein interactions. Screening by both proteomics62 and yeast two-hybrid63 approaches have revealed that TRPM1 forms complexes with nyctalopin, a protein essential for synaptic transmission to ON-BCs. Nyctalopin was also shown to engage in complex formation with mGluR6, and the elimination of either nyctalopin or mGluR6 prevented the synaptic targeting of TRPM1.62 These results indicate that nyctalopin is an essential component of the complex, possibly scaffolding the principal receptor mGluR6 and ion channel TRPM1 together at the dendritic tips of ON-BCs. Similarly, proteomic screening has identified orphan receptor GPR179 as a binding partner of RGS7 and RGS11.64 Indeed, the elimination of GPR179 prevented the synaptic targeting of both RGS7 and RGS11,64 providing a likely explanation that the synaptic deficits observed upon GPR179 loss in mice and humans with congenital night blindness are related to a disruption in the ability of the RGS complex to regulate mGluR6 signaling to TRPM1. In addition to GPR179, one of the RGS proteins, RGS11, is additionally controlled by R9AP,65,66 the same membrane anchor that associates with RGS9 in photoreceptors. Although the exact relationship between GPR179 and R9AP is not well understood, it is likely that RGS complexes in bipolar cells exist in several alternative configurations (e.g., RGS7/Gβ5/GPR179, RGS11/Gβ5/GPR179, and RGS11/Gβ5/R9AP).
Interestingly, there appears to be an additional layer of interactions in the scaffolding of the molecular components of the GPCR cascade in ON-bipolar neurons. Recent findings indicate that GPR179 also forms complexes with both TRPM1 and mGluR6,67 further bringing RGS proteins into close proximity of the key components of the cascade that they regulate. It appears that mGluR6 plays a central role in the web of protein-protein interactions, as its elimination prevents the targeting of most components and also decreases their proteolytic stability.68 Thus, multiple components are likely organized in a single macromolecular complex in which multiple signaling proteins are tightly integrated into a “signalosome.” Remarkably, this organization is reminiscent of the visual cascade in fly photoreceptors, whereby GPCR also signals to the TRP channel, which likely plays a critical role in ensuring the high spatiotemporal resolution of signaling.5
Similar Principles Guide the Organization of G Protein Cascades in the Central Nervous System
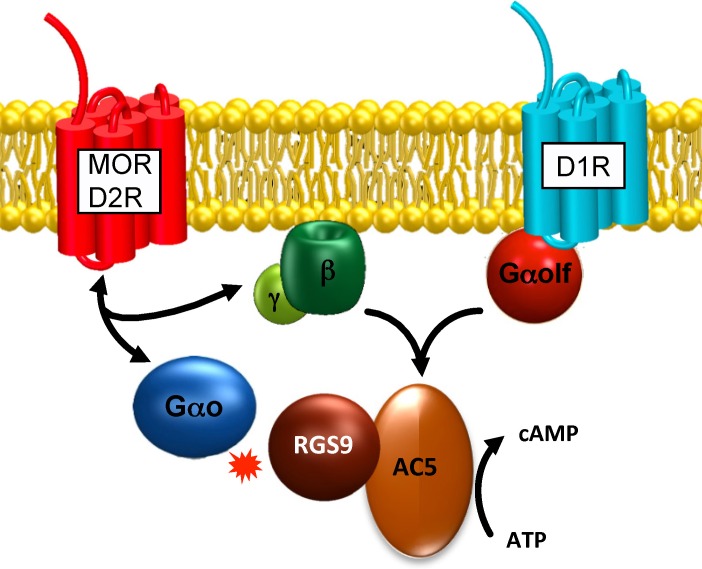
Advances in the understanding of the organization and functional regulation of G protein signaling pathways in the retina raise an inevitable question: How far do the lessons learned in this system extend? Furthermore, can they be applied for understanding the organization of GPCR pathways in the nervous system in general? We hope that recent studies provide a convincingly positive answer to these questions. Taking the first case in point, the key regulator of the phototransduction RGS9 complex is also prominently expressed in the brain region called the striatum.69–71 This structure is heavily involved in procedural learning, motivation, and reward valuation, essentially serving as the brain's reward and movement coordination center.72–74 The striatum integrates a vast number of incoming neurotransmitter inputs, many of which signal via GPCRs. Dysfunctions in the striatum are implicated in a range of neurological disorders as well as in drug addiction. In the striatal neurons, the G protein regulator RGS9 was shown to control signaling via two specific GPCRs in this region, μ-opioid75 and D2 dopamine receptors,76,77 which belong to the same class as rhodopsin. The elimination of RGS9 increases the behavioral sensitivity of animals to the μ-opioid receptor agonist morphine and exacerbates the dyskinetic manifestations associated with the long-term use of antipsychotics and L-DOPA, which act on the D2 receptor. Examining the role of RGS9 in controlling intracellular signaling, we found that it regulates the homeostasis of the second messenger cAMP (Fig. 2). Type 9 regulator of G protein signaling specifically associates with the effector enzyme that produces cAMP, adenylyl cyclase type 5 (AC5), and controls the extent to which Gβγ subunits released by the μ-opioid (and possibly D2R) receptor sensitize AC5 to the stimulatory effects of Gαs/olf.78 Chronic activation of opioid receptors has been described as resulting in the overactivation of AC5. During this process, also known as superactivation, the abrupt termination of signaling at opioid receptors, for example, through the introduction of an antagonist, leads to a surge in the cAMP concentration, which reflects homeostatic adaptation in the activity of AC5.79 An important role in this sensitization is played by the Gβγ subunits that potentiate AC5 activity. We have found that by controlling the lifetime of activated Gαo, which does not directly influence AC5 activity, RGS9 selectively blunts the stimulatory effect of Gβγ subunits on AC5. As a result, mice lacking RGS9 show significantly higher cAMP production in their striatal regions upon withdrawal from chronic morphine administration.78 This biochemical mechanism might help to explain the behavioral observations that are reported as more severe physical withdrawal signs as well as the higher degree of drug seeking in mice lacking RGS9.75,76 Interestingly, as in photoreceptors, the stability and membrane localization of RGS9 require its association with protein cofactors.80 In the striatum, RGS9 exists as a constitutive complex with the Gβ5 subunit, which is essential for its proteolytic stability. In addition, the expression level of RGS9 and its localization at the membrane are controlled by an R9AP-like protein called R7 family-binding protein, or R7BP.81,82 Overall, these studies identify two significant parallels with phototransduction. Similar molecular organization of the RGS complex: RGS9/Gβ5/R9AP in photoreceptors and RGS9/Gβ5/R7BP in striatal neurons and critical roles of these complexes in controlling the timing and extent of the second messenger homeostasis: cGMP in photoreceptors and cAMP in striatal neurons.
Figure 2.
Organization of the G protein signaling cascade in the medium spiny neurons of the striatum.
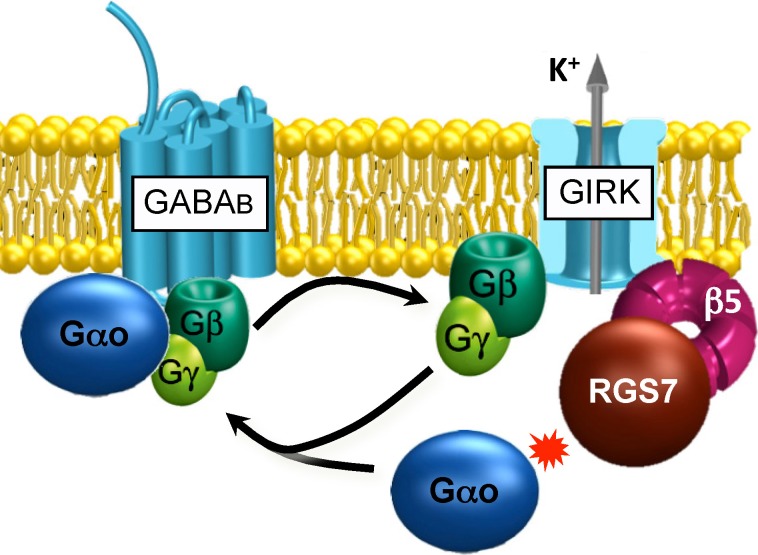
The second illustrative example is RGS7; much like RGS9, it is also expressed in the CNS, prominently in the hippocampus, the brain region associated with spatial learning and memory.83 Indeed, we have recently found that RGS7 plays an important role in contextual fear memories, as its knockout in mice disrupts their ability to remember the association of specific spatial cues with noxious stimulation.84 The elimination of RGS7 results in selective disruption in the inhibitory forms of synaptic plasticity, long-term depression (LTD) and depotentiation (DP). We have further found that, just like RGS9, RGS7 forms complexes with two subunits, R7BP and Gβ5, in the hippocampus. Curiously, Gβ5 was found to be essential not only for the proteolytic stability of the RGS7 complex but also for mediating complex formation with another effector enzyme, the ion channel known as G protein-activated inwardly rectifying K+ (GIRK) channel.85 The GIRK channel is gated by binding to the G protein βγ subunits released upon the activation of GABAB receptors, and Gβ5 mimics this interaction to recruit RGS7 to the complex (Fig. 3). The knockout of either RGS7 or Gβ5 dramatically slows down the GIRK channel deactivation kinetics, which results in a net increase in K+ efflux and a decrease in the resting membrane potential, thus decreasing the excitability of hippocampal neurons.84,85 Curiously, R7BP further shapes GIRK channel regulation via the RGS7 complex by recruiting the complex to the plasma membrane and adjusting the sensitivity of its regulation by GABAB receptor stimulation.84,86 Hence, the same parallels appear to also apply in explaining the organizational principles of the key GPCR signaling cascade in hippocampal pyramidal neurons: the essential regulation by RGS protein complexes that dictate the kinetics of effector ion channel activity, hence determining neuronal response properties.
Figure 3.
Organization of the G protein signaling cascade in the hippocampal CA1 pyramidal neurons.
Conclusions
The central lesson that we have learned thus far has been that despite their apparent difference, G protein signaling pathways in neurons of the retina, striatum, and hippocampus utilize similar regulatory principles based on the key involvement of RGS protein complexes, macromolecular scaffolding of the components, and cascade compartmentalization. We hope that these lessons can be further extended for a better understanding of the organization and physiological regulation of GPCR signaling systems across the nervous system.
Acknowledgments
I thank the scientists in my laboratory, whose diligent work made these studies possible: Yan Cao, Keqiang Xie, Ikuo Masuho, Cesare Orlandi, Ignacio Sarria, Olga Ostrovskaya and my wife and colleague Natalia Martemyanova. I am very grateful to the outstanding collaborations that my lab has had over the years with the laboratories of Alapakkam Sampath, Vladimir Kefalov, Kevin Wickman, Mark Thomas, Ronald Gregg, and Maureen McCall. Much of what I have learned about vision and photoreceptors came from my mentors Vadim Arshavsky, Edward Pugh and Marie Burns, to whom I am forever indebted. The work from my laboratory described in this lecture was funded by NIH Grants EY018139, DA036596, and DA026405.
The author alone is responsible for the content and writing of the paper.
Disclosure: K.A. Martemyanov, None
References
- 1. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002; 1: 727–730. [DOI] [PubMed] [Google Scholar]
- 2. Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995; 80: 249–257. [DOI] [PubMed] [Google Scholar]
- 3. Arshavsky VY, Lamb TD, Pugh EN Jr. G proteins and phototransduction. Annu Rev Physiol. 2002; 64: 153–187. [DOI] [PubMed] [Google Scholar]
- 4. Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Ret Eye Res. 2013; 36: 52–119. [DOI] [PubMed] [Google Scholar]
- 5. Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009; 139: 246–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005; 48: 387–401. [DOI] [PubMed] [Google Scholar]
- 7. Chen CK. The vertebrate phototransduction cascade: amplification and termination mechanisms. Rev Physiol Biochem Pharmacol. 2005; 154: 101–121. [DOI] [PubMed] [Google Scholar]
- 8. Burns ME, Pugh EN Jr. Lessons from photoreceptors: turning off g-protein signaling in living cells. Physiology (Bethesda). 2010; 25: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krispel CM, Chen D, Melling N, et al. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006; 51: 409–416. [DOI] [PubMed] [Google Scholar]
- 10. Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000; 403: 557–560. [DOI] [PubMed] [Google Scholar]
- 11. He W, Cowan CW, Wensel TG. RGS9, a GTPase accelerator for phototransduction. Neuron. 1998; 20: 95–102. [DOI] [PubMed] [Google Scholar]
- 12. Cowan CW, He W, Wensel TG. RGS proteins: lessons from the RGS9 subfamily. Prog Nucleic Acid Res Mol Biol. 2000; 65: 341–359. [DOI] [PubMed] [Google Scholar]
- 13. Makino ER, Handy JW, Li TS, Arshavsky VY. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein b subunit. Proc Natl Acad Sci U S A. 1999; 96: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen CK, Eversole-Cire P, Zhang HK, et al. Instability of GGL domain-containing RGS proteins in mice lacking the G protein b-subunit Gb5. Proc Natl Acad Sci U S A. 2003; 100: 6604–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He W, Lu L, Zhang X, et al. Modules in the photoreceptor RGS9-1.Gbeta 5L GTPase-accelerating protein complex control effector coupling, GTPase acceleration, protein folding, and stability. J Biol Chem. 2000; 275: 37093–37100. [DOI] [PubMed] [Google Scholar]
- 16. Skiba NP, Martemyanov KA, Elfenbein A, et al. RGS9-Gb5 substrate selectivity in photoreceptors—opposing effects of constituent domains yield high affinity of RGS interaction with the G protein-effector complex. J Biol Chem. 2001; 276: 37365–37372. [DOI] [PubMed] [Google Scholar]
- 17. Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci U S A. 2002; 99: 9755–9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martemyanov KA, Lishko PV, Calero N, et al. The DEP domain determines subcellular targeting of the GTPase activating protein RGS9 in vivo. J Neurosci. 2003; 23: 10175–10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keresztes G, Martemyanov KA, Krispel CM, et al. Absence of the RGS9/Gb5 GTPase-activating complex in photoreceptors of the R9AP knockout mouse. J Biol Chem. 2004; 279: 1581–1584. [DOI] [PubMed] [Google Scholar]
- 20. Arshavsky VY, Wensel TG. Timing is everything: GTPase regulation in phototransduction. Invest Ophthalmol Vis Sci. 2013; 54: 7725–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor WR, Smith RG. Transmission of scotopic signals from the rod to rod-bipolar cell in the mammalian retina. Vision Res. 2004; 44: 3269–3276. [DOI] [PubMed] [Google Scholar]
- 22. Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 1999; 22: 497–504. [DOI] [PubMed] [Google Scholar]
- 23. Nakajima Y, Iwakabe H, Akazawa C, et al. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993; 268: 11868–11873. [PubMed] [Google Scholar]
- 24. Masu M, Iwakabe H, Tagawa Y, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995; 80: 757–765. [DOI] [PubMed] [Google Scholar]
- 25. Dhingra A, Lyubarsky A, Jiang MS, et al. The light response of ON bipolar neurons requires Gao. J Neurosci. 2000; 20: 9053–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999; 19: 2938–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010; 107: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009; 106: 19174–19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009; 29: 6088–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Ret Eye Res. 2008; 27: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vardi N, Dhingra A, Zhang L, Lyubarsky A, Wang TL, Morigiwa K. Neurochemical organization of the first visual synapse. Keio J Med. 2002; 51: 154–164. [DOI] [PubMed] [Google Scholar]
- 32. Goodwin P. Hereditary retinal disease. Curr Opin Ophthalmol. 2008; 19: 255–262. [DOI] [PubMed] [Google Scholar]
- 33. Cideciyan AV, Jacobson SG. Negative electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1993; 34: 3253–3263. [PubMed] [Google Scholar]
- 34. Zeitz C, van Genderen M, Neidhardt J, et al. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci. 2005; 46: 4328–4335. [DOI] [PubMed] [Google Scholar]
- 35. Dryja TP, McGee TL, Berson EL, et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A. 2005; 102: 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura M, Sanuki R, Yasuma TR, et al. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis. 2010; 16: 425–437. [PMC free article] [PubMed] [Google Scholar]
- 37. van Genderen MM, Bijveld MM, Claassen YB, et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Audo I, Kohl S, Leroy BP, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998; 19: 264–267. [DOI] [PubMed] [Google Scholar]
- 40. Strom TM, Nyakatura G, Apfelstedt-Sylla E, et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998; 19: 260–263. [DOI] [PubMed] [Google Scholar]
- 41. Poopalasundaram S, Erskine L, Cheetham ME, Hardcastle AJ. Focus on molecules: nyctalopin. Exp Eye Res. 2005; 81: 627–628. [DOI] [PubMed] [Google Scholar]
- 42. Peachey NS, Ray TA, Florijn R, et al. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012; 90: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Audo I, Bujakowska K, Orhan E, et al. Whole-exome sequencing identifies mutations in gpr179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012; 90: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeitz C, Jacobson SG, Hamel CP, et al. Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2013; 92: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gregg RG, Mukhopadhyay S, Candille SI, et al. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003; 44: 378–384. [DOI] [PubMed] [Google Scholar]
- 46. Neuille M, El Shamieh S, Orhan E, et al. Lrit3 deficient mouse (nob6): a novel model of complete congenital stationary night blindness (cCSNB). PLoS One. 2014; 9: e90342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maddox DM, Vessey KA, Yarbrough GL, et al. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4 results in differences in retinal ganglion cell visual responses. J Physiol. 2008; 586: 4409–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dhingra A, Ramakrishnan H, Neinstein A, et al. Gbeta3 is required for normal light ON responses and synaptic maintenance. J Neurosci. 2012; 32: 11343–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007; 27: 14199–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004; 41: 431–443. [DOI] [PubMed] [Google Scholar]
- 51. Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004; 24: 5684–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009; 54: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Song JH, Song H, Wensel TG, Sokolov M, Martemyanov KA. Localization and differential interaction of R7 RGS proteins with their membrane anchors R7BP and R9AP in neurons of vertebrate retina. Mol Cell Neurosci. 2007; 35: 311–319. [DOI] [PubMed] [Google Scholar]
- 54. Morgans CW, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, Duvoisin RM. Gbeta5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. Eur J Neurosci. 2007; 26: 2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009; 29: 7753–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen FS, Shim H, Morhardt D, et al. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest Ophthalmol Vis Sci. 2010; 51: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009; 29: 7753–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang J, Jeffrey BG, Morgans CW, et al. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010; 51: 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cao Y, Masuho I, Okawa H, et al. Retina-specific GTPase accelerator RGS11/Gb5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009; 29: 9301–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shim H, Wang CT, Chen YL, et al. Defective retinal depolarizing bipolar cells (DBCs) in regulators of G-protein signaling (RGS) 7 and 11 double null mice. J Biol Chem. 2012; 287: 14873–14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc Natl Acad Sci U S A. 2012; 109: 7905–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J Neurosci. 2011; 31: 11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pearring JN, Bojang P Jr, Shen Y, et al. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J Neurosci. 2011; 31: 10060–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Orlandi C, Posokhova E, Masuho I, et al. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012; 197: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cao Y, Kolesnikov AV, Masuho I, Kefalov VJ, Martemyanov KA. Membrane anchoring subunits specify selective regulation of RGS9.Gbeta5 GAP complex in photoreceptor neurons. J Neurosci. 2010; 30: 13784–13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jeffrey BG, Morgans CW, Puthussery T, et al. R9AP stabilizes RGS11-G beta5 and accelerates the early light response of ON-bipolar cells. Vis Neurosci. 2010; 27: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Orlandi C, Cao Y, Martemyanov KA. Orphan receptor GPR179 forms macromolecular complexes with components of metabotropic signaling cascade in retina ON-bipolar neurons. Invest Ophthalmol Vis Sci. 2013; 54: 7153–7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dhingra A, Vardi N. “mGlu receptors in the retina” - WIREs membrane transport and signaling. Wiley Interdiscip Rev Membr Transp Signal. 2012; 1: 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rahman Z, Gold SJ, Potenza MN, et al. Cloning and characterization of RGS9-2: a striatal-enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999; 19: 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thomas EA, Danielson PE, Sutcliffe JG. RGS9: a regulator of G-protein signalling with specific expression in rat and mouse striatum. J Neurosci Res. 1998; 52: 118–124. [DOI] [PubMed] [Google Scholar]
- 71. Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997; 17: 8024–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008; 60: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003; 10: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Graybiel AM. The basal ganglia. Curr Biol. 2000; 10: R509–R511. [DOI] [PubMed] [Google Scholar]
- 75. Zachariou V, Georgescu D, Sanchez N, et al. Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A. 2003; 100: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rahman Z, Schwarz J, Gold SJ, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003; 38: 941–952. [DOI] [PubMed] [Google Scholar]
- 77. Cabrera-Vera TM, Hernandez S, Earls LR, et al. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A. 2004; 101: 16339–16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xie K, Masuho I, Brand C, Dessauer CW, Martemyanov KA. The complex of G protein regulator RGS9-2 and Gbeta(5) controls sensitization and signaling kinetics of type 5 adenylyl cyclase in the striatum. Sci Signal. 2012; 5:ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha I/O-coupled receptors. Pharmacol Ther. 2005; 106: 405–421. [DOI] [PubMed] [Google Scholar]
- 80. Martemyanov KA, Arshavsky VY. Biology and functional regulation of RGS9 isoforms. In: Fisher R. ed Molecular Biology of RGS Proteins. Waltham, MA: Academic Press; 2009: 205–227. [DOI] [PubMed] [Google Scholar]
- 81. Anderson GR, Semenov A, Song JH, Martemyanov KA. The membrane anchor R7BP controls the proteolytic stability of the striatal specific RGS protein, RGS9-2. J Biol Chem. 2007; 282: 4772–4781. [DOI] [PubMed] [Google Scholar]
- 82. Anderson GR, Lujan R, Semenov A, et al. Expression and localization of RGS9-2/G 5/R7BP complex in vivo is set by dynamic control of its constitutive degradation by cellular cysteine proteases. J Neurosci. 2007; 27: 14117–14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997; 17: 8024–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ostrovskaya O, Xie K, Masuho I, et al. RGS7/Gbeta5/R7BP complex regulates synaptic plasticity and memory by modulating hippocampal GABABR-GIRK signaling. eLife. 2014; 3: e02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie K, Allen KL, Kourrich S, et al. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010; 13: 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou H, Chisari M, Raehal KM, et al. GIRK channel modulation by assembly with allosterically regulated RGS proteins. Proc Natl Acad Sci U S A. 2012; 109: 19977–19982. [DOI] [PMC free article] [PubMed] [Google Scholar]