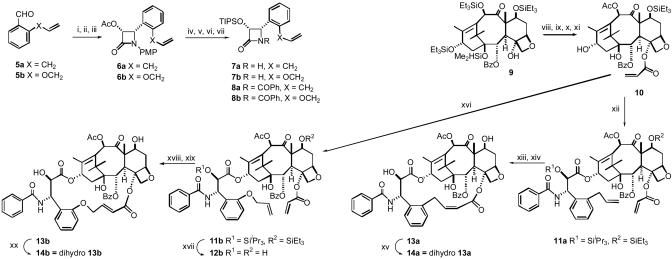
Fig. 1.
Synthetic scheme for the bridged paclitaxels 13a, 13b, 14a, and 14b. The reagents and conditions for the “a” series of β-lactams were similar to those below for the “b” series. Reagents and conditions for the “b” series β-lactams follow. i, 5b, p-MeOC6H4NH2, MgSO4, CH2Cl2, 100%. ii, CH3COOCH2COCl, Et3N, -78°C to room temperature (rt), 12 h, 85%. iii, Lipase (Amano PS), phosphate buffer, pH 7.2, CH3CN, 24 h, 98%. iv, 1M KOH, THF, 0°C, 100%. v, TIPSCl, imidazole, DMF, 94%. vi, CAN, CH3CN, -5°C, 62%. vii, PhCOCl, Et3N, DMAP, CH2Cl2, 95%. viii, LiHMDS, THF, 0°C, CH2—CHCOCl, 52%. ix, HF-pyridine, THF, 70%. x, CeCl3,Ac2O, THF, 96%. xi, Et3SiCl, imidazole, DCM, 72%. xii, 8a, NaH, THF, 0°C to rt, 24 h. xiii, (H2IMes)(PCy3)(CI)2Ru—CHPh, CH2Cl2, 3 h. xiv, HF-pyridine, 12 h. xv, H2,Pd/C(10%), 35 psi, 2.5 h. xvi, 8b, NaH, THF, 0°C to rt, 24 h, 50%. xvii, HF-Pyridine, THF, 81%. xviii, (H2IMes)(PCy3)(CI)2Ru—CHPh, CH2Cl2, 3 h, 64%. xix, HF-pyridine, 12 h, 98%. xx, H2, Pd/C(10%), 35 psi, 2.5 h, 96%.